Abstract
Eosinophilic pleural effusion (EPE) is defined as a pleural effusion that contains at least 10% eosinophils. EPE occurs due to a variety of causes such as blood or air in the pleural space, infection, malignancy, or an autoimmune disease. Undifferentiated connective tissue disease (UCTD) associated with eosinophilic pleural effusion is a rare condition generally characterized by the presence of the signs and symptoms but not fulfilling the existing classification criteria. We report a case involving a 67-year-old man with UCTD and EPE, who has been successfully treated with a single intrapleural corticosteroid injection.
Eosinophilic pleural effusion (EPE), defined as an effusion containing 10% or more eosinophils, accounts for 5% to 16% of exudative pleural effusions. It can be a manifestation of many different diseases, but one-third of the patients with EPE remain undiagnosed1. EPEs caused by an autoimmune disease are relatively rare (diseases such as rheumatoid arthritis, systemic lupus erythematosus [SLE], sarcoidosis, Churg-Strauss syndrome, and ulcerative colitis have been reported as the causes)1-8. The treatment of EPE involves the treatment of the underlying disease. There are data to support the view that systemic corticosteroids are effective in the treatment of EPEs associated with an autoimmune disease or induced by drugs9-11. Patients who present with nonspecific clinical or serological abnormalities and do not meet the diagnostic criteria for a specific rheumatic disease can be classified as having undifferentiated connective tissue disease (UCTD). However, no cases of EPEs associated with UCTD have been described so far. Intrapleural corticosteroid administration has been reported in a few cases of SLE-related and posttraumatic EPEs, but the results are inconsistent2,10. We present a case of an EPE associated with UCTD that was successfully treated with intrapleural corticosteroid administration.
A 67-year-old man was admitted with a 1-month history of increasing dyspnea of modified Medical Research Council grade 3. The patient had undergone mitral valve replacement 20 years earlier and had been treated for heart failure, diabetes, and hypertension. His medications included warfarin, digoxin, furosemide, losartan, isosorbide mononitrate, glimepiride, and thiotacid. He had no smoking history. At the time of hospitalization, the patient was alert and the following findings were noted in a physical examination: blood pressure, 140/60 mm Hg; pulse rate, 84 beats/min; respiratory rate, 20 breaths/min; and body temperature, 37.6℃. He had no history of chest trauma or any prominent abnormalities on chest, abdominal, and lower-extremity examinations. Other results of the physical examination were non-specific.
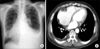
On a physical examination, decreased breath sounds were auscultated on the left lower thorax. A chest radiograph showed blunting of the left costophrenic angle (Figure 1A). Chest computed tomography images obtained after therapeutic thoracentesis revealed pleural thickening and calcification on the right posterior pleura but no nodule, mass, or lesion in the lung parenchyma showing in connective tissue disease (Figure 1B). Transthoracic echocardiography revealed enlargement of both atria, dilatation of the left-sided ventricle, and concentric left ventricular hypertrophy with left ventricle ejection fraction 57%. Mild mitral regurgitation and tricuspid regurgitation (grade II) were noted, but no definite change could be observed. There was no pericardial effusion or pericardial thickening, and no valvular vegetations were seen. Although a dose of diuretics had been added for several days, the amount of the left pleural effusion increased markedly, and the patient was referred to a pulmonologist. Laboratory examinations showed the following findings: white blood cell count, 9,300/mm3; hemoglobin, 11.7 g/dL; platelet count, 338,000/mm3; aspartate aminotransferase, 29 IU/L; alanine aminotransferase, 24 IU/L; total protein, 7.8 g/dL; blood urea nitrogen, 22 mg/dL; creatinine, 1.4 mg/dL; C-reactive protein, 8.3 mg/dL; and NT-pro B-type natriuretic peptide (BNP), 1,324 pg/mL. Arterial blood gas analysis in room air yielded results within normal ranges. Analysis of the pleural effusion revealed the following findings: color, yellow; pH, 7.4; specific gravity, 1.010; red blood cell count, 13,760/mm3; white blood cell count, 990/mm3 (neutrophils, 2%; lymphocytes, 29%; eosinophils, 34%); protein, 4.2 g/dL; lactate dehydrogenase, 384 U/L; glucose, 117 mg/dL; carcinoembryogenic antigen, 0.2 ng/mL; adenosine deaminase, 31 IU/L; negative acid-fast staining, culture, non-specific cytology, and negative results in real-time polymerase chain reaction for Mycobacterium tuberculosis. Diagnostic thoracentesis revealed a pleural effusion appearing as an exudate with an elevated eosinophil count (34%). Serological tests for parasites (Clonorchis sinesis, Paragonimus westermani, Cysticercus cellulosae, Sparganum) yielded negative results. Serological test for rheumatologic diseases and vasculitides yielded positive results for the antinuclear antibody (>1:1,280, speckled pattern) and the anti-SS-A/Ro (179.09 EU) and anti-SS-B/La antibodies (208.98 EU). Serological tests for rheumatoid factor (RF) (<20 IU/mL), anti-ds DNA, and complement (C3, C4) yielded negative results. Of the medications the patient was receiving, although warfarin was associated with EPE, he had been taking the drug without any side effects since the mitral valve replacement surgery. Therefore, we considered that the possibility of warfarin-associated EPE was low. The patient complained of xerostomia but not of xerophthalmia, Raynaud's phenomenon, skin lesion, or arthralgia. The patient's heart failure was well controlled; he showed no pitting edema, and his NT-pro BNP level had reduced from 2,419 pg/mL to 1,324 pg/mL in a 1-month period. EPE associated with UCTD was considered, and the patient was discharged after 1 L of excess fluid was removed by therapeutic thoracentesis. About 2 weeks later, his dyspnea worsened and the left pleural effusion increased again. Repeated therapeutic thoracentesis temporarily relieved the symptoms, but they aggravated again within 2 weeks. The administration of systemic corticosteroid was considered but was contraindicated by chronic anticoagulation therapy, uncontrolled blood sugar levels, and the patient's age.
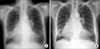
Because we were reluctant to administer systemic corticosteroid therapy, a single dose of 125 mg methylprednisolone was injected directly into the pleural space. A chest X-ray image obtained 2 weeks later showed a marked decrease in the pleural effusion (Figure 2A). No recurrence was observed during a 1-year follow-up, and serological tests for autoantibodies yielded similar results to those obtained in the previous year (Figure 2B).
EPEs, defined as effusions with 10% or more eosinophils, typically account for 5% to 16% of exudative pleural effusions1. EPEs can be caused by various conditions, including air or blood in the pleural space, malignancy, infection, pulmonary embolism, drug reactions, and autoimmune diseases7. EPEs related to UCTD have not been reported in the literature so far.
UCTD has been defined as a condition characterized by the presence of the signs and symptoms of a connective tissue disease but not meeting the existing classification criteria4. Our patient had xerostomia but did not have xerophthalmia, skin rash, or arthralgia. The patient's samples were strongly positive for antinuclear, anti-SS-A/Ro, and anti-SS-B/La antibodies but were negative for RF (<20) and anti-ds DNA. The definition of UCTD includes a wide spectrum of diseases ranging from organ-dominant conditions to simplified conditions, to early CTDs or mild forms of CTDs4. Antibodies to nuclear antigens (ANAs) are detected in the serum of 50-97% of the patients with UCTD. Most of them are directed to Ro/SSA or to U1 ribonucleoprotein (RNP) antigens12. When the presence of ANA is required for the definition UCTD, the condition usually presents with a single antibody specificity, with anti-Ro/SSA and anti-RNP antibodies being most frequently observed (8-30% and 10-30% of the patients, respectively)4. In the present case, the patient' symptoms and signs were not specific enough to make a definitive diagnosis of CTD. This condition can be classified as anti-Ro/SSa-positive UCTD because of ANA with Ro/SSA positive antibodies, which do not fulfill the connective tissue disease classification. Therefore, he could be diagnosed with EPE related to UCTD.
The treatment of EPE involves the treatment of the underlying disease1. Systemic corticosteroids are effective for the treatment of EPE associated with acute or chronic eosinophilic pneumonia and hypereosinophilic syndrome1. After the administration of 20 mg/day paramethasone, the pleural effusion decreased in progressive systemic sclerosis-polymyositis overlap syndrome with EPE5.
The therapeutic options for refractory massive pleural effusion in SLE include 7 different types of therapy10. When refractory pleural effusion is related to lupus exacerbation, the treatment of choice is systemic (such as an immunosuppressive therapy with high-dose steroids and cyclophosphamide). Intrapleural steroid injections were reported in 3 patients at the doses of 100 to 240 mg methylprednisolone or 80 mg triamcinolone, but they were ineffective. In contrast, 2 cases with post-traumatic pleural effusion were successfully treated with intrapleural steroids2. In the present case, we decided to administer intrapleural corticosteroid therapy because of the patient's age and his history of diabetes and heart failure. As mentioned above, a single dose of 125 mg methylprednisolone was injected into the intrapleural space, which resulted in a dramatic improvement in the left pleural effusion.
The differentiation of UCTD into a specific connective tissue disease usually occurs within 5 years after the disease onset. UCTD may transform into SLE, systemic sclerosis, primary Sjogren's syndrome, mixed connective tissue disease, systemic vasculitis, polydermatomyositis, or rheumatoid arthritis4. During follow-up, our patient remained asymptomatic and serological tests yielded positive results. We did not determine whether UCTD in our patient transformed into any of connective tissue diseases.
To our knowledge, this is the first report of EPE in a patient with UCTD. After corticosteroid administration, the pleural effusion rapidly decreased.
The incidence of EPE in patients with autoimmune diseases is reported to be as low as 4%5. However, if the cause of EPE is unknown, it may be considered to be related to UCTD. Intrapleural corticosteroid may be considered as one of the therapeutic options in patients in whom the administration of systemic corticosteroid is not possible.
Figures and Tables
References
1. Kalomenidis I, Light RW. Eosinophilic pleural effusions. Curr Opin Pulm Med. 2003; 9:254–260.
2. Ishiura Y, Fujimura M, Nakamura N, Jokaji H, Minami S, Matsuda T. Intrapleural corticosteroid injection therapy for post-traumatic eosinophilic pleural effusion. Respir Med. 1996; 90:501–503.
3. Rubins JB, Rubins HB. Etiology and prognostic significance of eosinophilic pleural effusions: a prospective study. Chest. 1996; 110:1271–1274.
4. Mosca M, Tani C, Carli L, Bombardieri S. Undifferentiated CTD: a wide spectrum of autoimmune diseases. Best Pract Res Clin Rheumatol. 2012; 26:73–77.
5. Maeshima E, Nishimoto T, Yamashita M, Mune M, Yukawa S. Progressive systemic sclerosis-polymyositis overlap syndrome with eosinophilic pleural effusion. Rheumatol Int. 2003; 23:252–254.
6. Ferreiro L, San Jose E, Gonzalez-Barcala FJ, Alvarez-Dobano JM, Golpe A, Gude F, et al. Eosinophilic pleural effusion: incidence, etiology and prognostic significance. Arch Bronconeumol. 2011; 47:504–509.
7. Oba Y, Abu-Salah T. The prevalence and diagnostic significance of eosinophilic pleural effusions: a meta-analysis and systematic review. Respiration. 2012; 83:198–208.
8. Krenke R, Nasilowski J, Korczynski P, Gorska K, Przybylowski T, Chazan R, et al. Incidence and aetiology of eosinophilic pleural effusion. Eur Respir J. 2009; 34:1111–1117.
9. Samman YS, Wali SO, Abdelaal MA, Gangi MT, Krayem AB. Chronic eosinophilic pneumonia presenting with recurrent massive bilateral pleural effusion: case report. Chest. 2001; 119:968–970.
10. Breuer GS, Deeb M, Fisher D, Nesher G. Therapeutic options for refractory massive pleural effusion in systemic lupus erythematosus: a case study and review of the literature. Semin Arthritis Rheum. 2005; 34:744–749.
11. Felz MW, Haviland-Foley DJ. Eosinophilic pleural effusion due to dantrolene: resolution with steroid therapy. South Med J. 2001; 94:502–504.
12. Cavazzana I, Franceschini F, Belfiore N, Quinzanini M, Caporali R, Calzavara-Pinton P, et al. Undifferentiated connective tissue disease with antibodies to Ro/SSa: clinical features and follow-up of 148 patients. Clin Exp Rheumatol. 2001; 19:403–409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download