Abstract
Sarcoidosis, a systemic granulomatous disease of unknown etiology. The presentation of sarcoidal granuloma in neck nodes without typical manifestations of systemic sarcoidosis is difficult to diagnose. We describe the case of a 37-year-old woman with an increasing mass on the right side of neck. The excisional biopsy from the neck mass showed noncaseating epithelioid cell granuloma of the lymph nodes. No evidence of mycobacterial or fungal infection was noted. Thoracic evaluations did not show enlargement of mediastinal lymph nodes or parenchymal abnormalities. Immunohistochemistry showed abundant expression of tumor necrosis factor-α in the granuloma. However, transforming growth factor-β was not expressed, although interleukin-1β was focally expressed. These immunohistochemical findings supported characterization of the granuloma and the diagnosis of sarcoidosis. Sarcoidosis can present with cervical lymph node enlargement without mediastinal or lung abnormality. Immunohistochemistry may support the diagnosis of sarcoidosis and characterization of granuloma.
Sarcoidosis is a multi-system chronic inflammatory condition of unknown etiology. It usually presents with bilateral hilar lymphadenopathy, pulmonary infiltration, and skin and ocular lesions. Sarcoidosis is characterized by noncaseating epithelioid cell granulomas in affected organs, particularly in the lung, hilar lymph nodes (LNs), skin, and eyes1. Sarcoidosis can affect individuals of any race, sex, and age, but it commonly affects young to middle-aged adults. Thoracic radiologic abnormalities are common, and most of the morbidity and mortality associated with sarcoidosis involves the lung.
The diagnosis of sarcoidosis is established on the basis of compatible clinical and radiologic findings, supported by histologic evidence of noncaseating epithelioid-cell granulomas in one or more organs in the absence of organisms or particles2. Biopsy is recommended for all patients presumed to have sarcoidosis, except for those with Lofgren's syndrome2. Pathologists can identify granulomas, but the diagnosis should not be based on pathological findings alone.
Clinically, many conditions result in sarcoid-like granulomas that may be interpreted as a local reaction to a malignancy, a noncaseating reaction to a focus of caseating tuberculosis, or as another inflammatory disease3. These known granulomatous diseases should be excluded in patients without typical symptoms of sarcoidosis. The most common clinical features are respiratory symptoms, fatigue, night sweats, weight loss, erythema nodosum2. However, 50% of cases of sarcoidosis are asymptomatic, with abnormalities detected incidentally during chest radiography. Bilateral hilar lymphadenopathy is the earliest and most common manifestation of sarcoidosis. Peripheral lymphadenopathy is less likely to present first4.
Currently, the presentation of sarcoidal granuloma in neck nodes without typical manifestation of systemic sarcoidosis remains a diagnostic challenge. We present a case of cervical LN enlargement without mediastinal or lung parenchymal abnormality. Immunohistochemistry supported a diagnosis of sarcoidosis and excluded other granulomatous disorders.
A 37-year-old female patient presented with a palpable non-tender mass on the right side of her neck for 1 month. She had no other symptoms such as coughing, sputum production, rhinorrhea, arthralgia, fever, body weight loss, or dyspnea. Physical examination revealed a palpable non-tender mass on the right side of her neck but no splenomegaly or hepatomegaly. She had no uveitis. The erythrocyte sedimentation rate was 11 mm/hr (normal range for female individuals, <25 mm/hr), with a C-reactive protein level of 0.14 mg/dL (normal range, 0-0.5 mg/dL) and a serum angiotensin converting enzyme level of 23.9 U/L (normal range, 9.0-47.0 U/L). Neck computed tomography showed an approximately 50-mm abnormal LN at level V on the right and an approximately 28-mm abnormal LN at level II on the right (Figure 1). Multiple enlarged LNs were noted at levels II, III, IV, and V on the right. Chest computed tomography showed no abnormal nodular infiltration at either lung, but showed enlarged LNs in the left supraclavicular and left axillar areas.
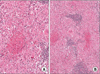
Fine needle aspiration from the right cervical LN revealed granulomatous inflammation with focal and central necrosis. Acid fast bacilli (AFB) stain was negative, and polymerase chain reaction for mycobacterium was also negative. Excisional biopsy of right neck LN (level V) was performed. A 4×2.5-cm pink ovoid granulomatous mass was observed at the posterolateral side of the sternocleidomastoid muscle. The mass was well encapsulated, with mild adhesion to the surrounding soft tissue. Excisional LN biopsy from the right side of the neck revealed granulomatous inflammation with focal and central necrosis (Figure 2). AFB stain showed no AFB positive bacilli in the excisional LN. Polymerase chain reaction for mycobacterium tuberculosis and non-tuberculous mycobacterium were again negative in the excisional LN. Clinically, we excluded all other exogenous conditions that may cause granulomatous inflammation in the lung, and sarcoidosis was diagnosed.
Immunohistochemistry showed diffuse high expression of tumor necrosis factor-α (TNF-α) in epithelioid cells and multinucleated giant cells. Interleukin (IL)-1β was focally expressed in the granuloma. However, transforming growth factor-β (TGF-β) was not expressed in the granulomatous lesion (Figure 3). We administered 10 mg/day prednisolone. Follow-up chest computed tomography after 3 months showed no interval change from baseline. The patient had no symptoms and no palpable LNs at the 6-month follow-up visit.
Sarcoidosis is an inflammatory systemic disease characterized by noncaseating granulomas composed of epithelioid giant cells, macrophages, and lymphocytes5.
Cervical lymphadenopathy as the sole and initial presentation of sarcoidosis is rare, thereby adding to the difficulty in diagnosis. Furthermore, sarcoidosis presenting as isolated asymptomatic neck mass was not reported yet. In this case, she did not recognize her neck mass until growing about 3 cm in diameter. From November 1996 to June 1999, 10 medical centers (in the US) and 1 coordinating center conducted A Case Control Etiologic Study of Sarcoidosis (ACCESS) including 736 patients. In total, 699 patients showed thoracic involvement, 368 had concomitant extra-thoracic disease, and 6 had isolated head and neck sarcoidosis6.
A diagnosis of sarcoidosis is established on the basis of compatible clinical and radiologic findings and is supported by histologic evidence of noncaseating epithelioid-cell granulomas in one or more organs in the absence of organisms or particles. Sarcoidal granulomas have no unique histologic features to differentiate them from other granulomas2. Chronic exposure to inflammatory stimuli and/or foreign bodies may contribute to the development of granulomatous disease7. Therefore, other important differential diagnoses of granulomatous disease (e.g., Crohn's disease, toxoplasmosis, lymphoma, and vasculitis) must be excluded. The pathological findings of sarcoidosis are granulomas, which are usually non-necrotizing, but occasionally, necrosis can be observed. Diagnosis of sarcoidosis involves exclusion of the diagnoses of the other granulomatous diseases, and in particular, tuberculosis.
Immunohistochemical staining is helpful in the diagnosis of granulomatous disease. The presence of CD4 T cells that interact with antigen-presenting cells indicates initiation of the formation and maintenance of granulomas. The triggering antigens activate selective T-cell clones that differentiate into type 1 helper T cells. Interferon-α and IL-2 are mainly secreted, and production of TNF-α is increased through macrophage activation. Pulmonary fibrosis occurs after a shift in content from Th1 cytokines to Th2 cytokines (mainly IL-4, IL-10, and IL-13).
TNF-α is a key factor in sarcoidosis, both for initiation and at the chronic stage8. Cervical lymphadenopathy is difficult to distinguish from other granulomatous diseases and malignancy. Therefore, TNF-α can be an adjuvant indicator of sarcoidosis. In the present case, abundant expression of TNF-α was noted in the granuloma, supporting the diagnosis of sarcoidosis. TNF-α is also thought to play a major role in the proliferation and spontaneous activity of macrophages and T-lymphocytes at the sites of inflammation and in the formation of noncaseating granuloma9. Therefore, TNF-α inhibitors may have a therapeutic effect on granulomatosis, such as that in the present case8.
In patients with pulmonary sarcoidosis, TGF-β is abundantly expressed in lung tissues and is localized to the epithelioid histiocytes within non-necrotizing granulomas10. However, TGF-β was not expressed in the granuloma in the present case. It is uncertain, therefore, whether TGF-β contributes only to the formation of granuloma in the lung parenchyma.
IL-1β released from alveolar macrophages in pulmonary tuberculosis is important in host defense against mycobacterial infection. The release of IL-1β was greater from alveolar macrophages of tuberculosis patients than from those of normal subjects11.
However, IL-1β was not expressed in the alveolar macrophages of patients with sarcoidosis12. In the present case, IL-β was focally expressed in the granuloma, thereby supporting a diagnosis of sarcoidosis rather than tuberculosis.
Sarcoidosis can present with cervical LN enlargement without mediastinal or lung abnormality. Immunohistochemistry may support the diagnosis of sarcoidosis and characterization of granulomas. Furthermore, TNF-α inhibitors could be an option for treating sarcoidosis, as in the present case.
Figures and Tables
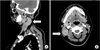 | Figure 1Sagittal (A) and axial (B) images of enhanced neck computed tomography (CT). (A) Sagittal images of enhanced neck CT show multiple enlarged lymph nodes at levels II, III, IV, and V. (B) Axial images of enhanced neck CT show multiple enlarged lymph nodes at levels II, III, IV, and V. The short diameter of the largest lymph node is approximately 3 cm (arrow). |
References
1. Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003; 361:1111–1118.
2. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153–2165.
3. Giuffrida TJ, Kerdel FA. Sarcoidosis. Dermatol Clin. 2002; 20:435–447.
4. Handa R, Aggarwal P, Wali JP, Wig N, Dinda AK, Biswas A. Sarcoidosis presenting with peripheral lymphadenopathy. Sarcoidosis Vasc Diffuse Lung Dis. 1998; 15:192.
5. Saltini C, Crystal RG. Pulmonary sarcoidosis: pathogenesis, staging and therapy. Int Arch Allergy Appl Immunol. 1985; 76:Suppl 1. 92–100.
6. Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004; 170:1324–1330.
7. Knopf A, Lahmer T, Chaker A, Stark T, Hofauer B, Pickhard A, et al. Head and neck sarcoidosis, from wait and see to tumor necrosis factor alpha therapy: a pilot study. Head Neck. 2013; 35:715–719.
8. Daien CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford). 2009; 48:883–886.
9. Fehrenbach H, Zissel G, Goldmann T, Tschernig T, Vollmer E, Pabst R, et al. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur Respir J. 2003; 21:421–428.
10. Limper AH, Colby TV, Sanders MS, Asakura S, Roche PC, DeRemee RA. Immunohistochemical localization of transforming growth factor-beta 1 in the nonnecrotizing granulomas of pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994; 149:197–204.
11. Kuo HP, Wang CH, Huang KS, Lin HC, Yu CT, Liu CY, et al. Nitric oxide modulates interleukin-1beta and tumor necrosis factor-alpha synthesis by alveolar macrophages in pulmonary tuberculosis. Am J Respir Crit Care Med. 2000; 161:192–199.
12. Bost TW, Riches DW, Schumacher B, Carre PC, Khan TZ, Martinez JA, et al. Alveolar macrophages from patients with beryllium disease and sarcoidosis express increased levels of mRNA for tumor necrosis factor-alpha and interleukin-6 but not interleukin-1 beta. Am J Respir Cell Mol Biol. 1994; 10:506–513.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download