Abstract
Although the relationship between malignancy risk with systemic sclerosis (SSc) has been inconclusive, there are some previous studies for a positive correlation. Most patients with SSc have some degree of lung parenchymal involvement in the form of interstitial thickening and fibrosis. Interstitial lung disease is the most common pulmonary manifestation of SSc. Interstitial lung disease following chemotherapy (5-fluorouracil, leucovorin, and oxaliplatin [FOLFOX]) is an uncommon life-threatening complication and it is induced by oxaliplatin. We report a case of multiple cancers in a patient with SSc and aggravated interstitial lung disease by chemotherapy.
Systemic sclerosis (SSc) is a heterogeneous disease with the distinct characteristic of abnormal immune system and over-production of collagen due to endothelial cell dysfunction and fibroblast control disorder. These changes cause gradual fibrosis of internal organs and skin cells, thereby leading to potential death and organ failure. The cause of the disease is unknown, but it is generally considered that the disease brings changes to genetic and environmental factors, as well as patient's susceptibility1.
SSc patients with interstitial lung disease generally have worse prognosis than those without the disease and thus have a higher level of mortality. Rosenthal said that the relative risk of all types of cancer among 233 SSc patients was 2.5 times higher than those without SSc. He stated that the risk of skin and hematological cancer incidence rate is particularly higher2. During retrospective study with 112 participants, Kang et al.3 mentioned that the SSc patients had 4.2 times higher incidence rate of cancer compared to general population. Also, he said that lung cancer is the most common cancer.
On the other side, 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), when prescribed as adjuvant chemotherapy, is effective in improving disease-free survival rate of colorectal cancer patients4,5. The side effects from FOLFOX are peripheral neuritis, nausea, vomiting and diarrhea, etc. The uncommon side-effect is interstitial lung disease. According to study results, occurrence rate of interstitial lung disease from FOLFOX is 0% to 4.2%, and it is induced by oxaliplatin6.
In regard to this point, we have seen a case in which interstitial lung diseases from SSc patients have aggravated after FOLFOX chemotherapy. Such interstitial lung diseases are also related to lung cancer, colorectal cancer and thyroid cancer. We hereby write a report on this particular case along with other related literature studies.
A 52-year-old female patient was referred to our hospital because of narrow feces. The patient had the symptom of narrow feces. During the first visit, she was diagnosed with sigmoid colon and was later hospitalized for surgery. The patient has been taking meloxicam (15 mg) for her knee pain in the past 3 years (prescribed by private clinic). No particular family factor is observed.
The patient only showed chronic ill looking apperance when she was first hospitalized. The blood pressure level was 118/83 mm Hg with pulse rate of 90/min, and respiratory rate of 20/min, body temperate was 36.9℃. There was no findings on conjunctiva blanching reaction and scleral icterus. The lung sound was clear. The abdomen was flat with normal bowel sound. There was no abdomen mass and tenderness. Digital rectal examination showed tarry stool.
Peripheral blood test when the patient was first hospitalized: The white blood cell was 6,460/mm3 (neutrophil 63.5%, lymphocyte 25.1%), hemoglobin 13.1 g/dL, platelet 169,000/mm3. Serum biochemistry showed the blood urea nitrogen level of 9.0 mg/dL and creatine 0.65 mg/dL, total protein 7.8 g/dL, albumin 3.7 mg/dL, aspartate aminotransferase/alanine aminotransferase 25/17 IU/L, a total bilirubin of 0.43 mg/dL, sodium 138 mEq/L, potassium 4.1 mEq/L, lactate dehydrogenase 427 IU/L, C-response protein 0.73 mg/L (range, <5 mg/L), carcinoembryonic antigen 2.49 ng/mL (range, 0.5 ng/mL), cancer antigen 19-9 24.15 U/mL (range, <39 U/mL).
No particular manifestation during simple chest radiography. Simple abdominal radiography showed a considerable amount of fecal impact. Abdomen computed tomography (CT) scan showed about 4.3 cm sized eccentric wall thickening with pericolic infiltration in sigmoid colon (Figure 1). Chest CT scan showed a ground glass opacity and reticular opacities in both lower lobes on admission (Figure 2). Positron emission tomography (PET) CT showed an immensely localized fluorodeoxyglucose (FDG) uptake (SUVmax 8.4) in sigmoid colon. There was mild crystalline FDG uptake around the intestine and on the frontal sacrum. There was severe FDG uptake on both sides of thyroid lobes (Rt, 8.6; Lt, 6.9) and mild FDG uptake at the lower part of pleural in the lower lobes on both sides of lung.
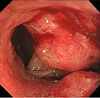
Colonoscopy has proceeded up to the end of ileum and removed 3 polyps (0.4 cm/0.3 cm/0.2 cm) in the middle of ascending colon. The examination also found 3 cm sized circumferential ulcerative and fungating mass at anal verge 17.20 cm (Figure 3).
Laparoscopic anterior resection was performed because there was no distant metastasis on PET CT. The size of tumor was 8.3×5.3×1.5 cm and it has infiltrated up to subseroa (pT3) in depth. The length of resection margin was 12.5 cm to upper end and 5.5 cm bottom end and 11 cm towards mesentery. There was no lymph node metastasis. No infiltration to adjacent nerves and blood vessels. But, there was infiltration to lymphatic channel.
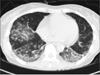
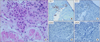
Afterwards, FOLFOX was performed 5 times as adjuvant chemotherapy. The patient complained of dry cough in the process of 4th chemotherapy. The patient had exertional dyspnea one month prior to the 5th chemotherapy. In comparison with the first observation, chest CT showed the interval progression of the ground glass opacity in both lower lobes (Figure 4). Pulmonary function test showed restrictive lung disease manifestations with forced vital capacity (FVC) 53%, forced expiratory volume in one second (FEV1) 64%, FEV1/FVC 90%, DLco 44%. Brochoalverolar lavage showed the result of neutrophil 2%, lymphocyte 10%, eosinophil 17%, macrophage 68%, and mesothelial 3%. Chest CT found no legional mass. PET CT did not find any suspicious FDG uptake. However, transbronchial lung biopsy (TBLB) at Rt. lower lung showed adenocarcinoma (poorly differentiated, cytokeratin [CK]-7[+]/CK-20[-]/thyroid transcription factor 1[+]/CDX-2[-]) (Figure 5).
After seeing strong FDG uptake at both thyroid lobes from PET CT (Rt, 8.6; Lt, 6.9) and the test result of T3 0.86 ng/mL (range, 0.6-1.8 ng/mL), T4 7.29 µg/dL (range, 5.4-11.7 µg/dL), free T4 0.99 ng/dL (range, 0.89-1.76 ng/dL), thyroid-stimulating hormone (TSH) 2.61 mIU/L (range, 0.55-4.78 mIU/L), TSH-R-Ab 0.23 IU/L (range, 0-9 IU/L), anti-thyroglobulin-Ab 349.78 U/mL (range, <115 U/mL), thyroid peroxidase Ab 1,823.09 U/mL (range, <34 U/mL), Tg 186.4 ng/mL (range, 1.3-31.8 ng/mL), ultrasonography was given. There was 5×7×8 mm sized ganglion in the middle of right lobe. Also, malignant nodule was found. Fine needle aspiration biopsy (FNAB) was given afterwards and the patient was diagnosed with papillary thyroid carcinoma.
Autoimmune examination was given and the result was antinuclear antibody positive (type: nucleolar and homogeneous, 1:1,600), rheumatoid arthritis factor 36.7 (range, 0.20), anti-cyclic citrullinated peptide Ab (-), anti-neutrophil cytoplasmic antibody (-), anti-DNA Ab (-), C3 100 mg/dL (range, 76.139 mg/dL), C4 19.5 mg/dL (range, 12.37 mg/dL), anti-SS-A (+), anti-RO (+), anti Scl-70 (+), anti-ribosomal-P (+). The patient was diagnosed with SSc.
After adjuvant chemotherapy, chest CT showed the aggravation of interstitial lung disease caused by SSc. The patient's condition was improved after 14 days of prednisolone 60 mg prescription. As of now, the patient is being monitored on an outpatient basis.
The above patient was first diagnosed with sigmoid colon cancer before receiving laparoscopic anterior resection. During the 4th adjuvant chemotherapy, the symptoms of dry cough and exertional dyspnea led to close examination with chest CT scan. Ground glass opacity was found at the both low lobes of lungs and the aggravation of interstitial lung disease due to FOLFOX. Autoimmune study was given to exclude other cause of the interstitial lung disease from chest CT result. The patient was diagnosed with SSc afterwards. From TBLB, which was performed for the purpose of diagnosing interstitial lung disease in relation to SSc, but the patient was diagnosed with adenocarcinoma. Papillary thyroid cancer was diagnosed from FNAB due to an increase in FDG uptake from PET CT.
Most patients with SSc previously had lung infiltration and they are classified into two categories: interstitial lung disease and pulmonary arterial hypertension. 90% of interstitial lung disease patients are confirmed with systemic SSc during autopsy. And, 85% of them are also diagnosed by high resolution computed tomography1,7.
In retrospective study with 965 SSc participants, the 9-year survival rate was 72% for those who did not have disease. However, the survival rate for SSc patients with severe interstitial lung disease was only 30%8.
Starting with Zatuchni's report in 1953 about pachydermatosis patients with alveolar cell carcinoma and its relevance to cancer, many studies had reported about the relevance with lung, breast cancer and hematologic malignancy9,10. Siau, in his cohort study11 with 68 SSc patients in eastern/western areas of England, announced the result that their relative risk of cancer is 3.15 times higher. Hematologic malignancy was the most prevalent cancer out of other types.
The relationship between SSc and cancer is not as clear as other connective tissue diseases7. And, depending on various reports, the specific types of cancers with a higher level of relevancy are also different. But, Kang et al.3 recently stated in his retrospective study with 112 participants that the incidence rate of patients with SSc is 4.2 times higher than that of general population. He said that the most common type was lung cancer2,11. This study show a patient with SSc who was simultaneously diagnosed with sigmoid colon cancer, lung cancer and thyroid cancer. This can be an exemplary case to demonstrate the high level of relevancy between SSc and cancer. The causality between SSc and cancer is not clear. But we can think of 1) chronic organ tissue damage and 2) control disorder within intercellular signal transduction pathway as potential causes. There are two examples: pulmonary fibrosis in relation to SSc as a cause of lung cancer, and control disorder for signal pathway between transforming growth factor-β and downstream SMAD as a cause for breast cancer, skin cancer and hematologic malignancy7.
Most patients with SSc have experienced lung infiltration, and interstitial infiltration of lung is usually found from about 85% of them through chest CT scan2. From the case study, the patient did not show difficulty in breathing in the beginning stage of chemotherapy. But we were able to find ground glass opacity and reticular opacities from chest CT.
In Shimura et al.'s study6, the average treatment frequency of FOLFOX before leading to interstitial lung disease was about 10 times (range, 2.17 times). And the average dose for oxaliplatin was 850 mg/m2 (range, 170.1,445 mg/m2). From this case study, the symptom of interstitial lung disease from SSc occurred during adjuvant chemotherapy at 4th FOLFOX when the patient was confirmed with the aggravation of interstitial lung disease. We have assessed that this resulted not from deterioration of SSc but from interstitial lung disease 5F-fluorouracil caused by FOLFOX treatment or leucovorin have no case of triggering interstitial lung disease. So, it is assessed that it came from oxaliplatin. A low dosage of steroid was prescribed for the occurrence of interstitial lung disease or when it is not severe. When the disease is severe, methylprednisolone is prescribed with a high dosage of steroid6. The 2 weeks of prednisolone 60 mg also improved the patient's condition in this case study.
In conclusion, there is a case in which interstitial lung diseases from SSc patients have aggravated after chemotherapy. Such interstitial lung diseases are also related to lung cancer, including involved with other cancers.
Figures and Tables
Figure 1
Abdomen and pelvis computed tomography shows about 4.3 cm sized eccentric wall thickening with pericolic infiltration in sigmoid colon.

Figure 2
Chest computed tomography shows ground glass opacity and reticular opacities in both lower lobes on admission.
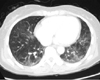
Figure 3
Colonoscopy shows about 3 cm sized circumferential ulcerative and fungating mass at anal verge 17-20 cm.
References
1. Hassoun PM. Lung involvement in systemic sclerosis. Presse Med. 2011; 40(1 Pt 2):e3–e17.
2. Rosenthal AK, McLaughlin JK, Gridley G, Nyren O. Incidence of cancer among patients with systemic sclerosis. Cancer. 1995; 76:910–914.
3. Kang KY, Yim HW, Kim IJ, Yoon JU, Ju JH, Kim HY, et al. Incidence of cancer among patients with systemic sclerosis in Korea: results from a single centre. Scand J Rheumatol. 2009; 38:299–303.
4. Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350:2343–2351.
5. Jeon HJ, Woo JH, Lee HY, Park KJ, Choi HJ. Adjuvant chemotherapy using the FOLFOX regimen in colon cancer. J Korean Soc Coloproctol. 2011; 27:140–146.
6. Shimura T, Fuse N, Yoshino T, Minashi K, Tahara M, Doi T, et al. Clinical features of interstitial lung disease induced by standard chemotherapy (FOLFOX or FOLFIRI) for colorectal cancer. Ann Oncol. 2010; 21:2005–2010.
7. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, et al. Harrison's principles of internal medicine. 18th ed. New York: McGrow Hill;2012.
8. Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000; 43:2437–2444.
9. Lazarus MN, Robinson D, Mak V, Moller H, Isenberg DA. Incidence of cancer in a cohort of patients with primary Sjogren's syndrome. Rheumatology (Oxford). 2006; 45:1012–1015.
10. Pearson JE, Silman AJ. Risk of cancer in patients with scleroderma. Ann Rheum Dis. 2003; 62:697–699.
11. Siau K, Laversuch CJ, Creamer P, O'Rourke KP. Malignancy in scleroderma patients from south west England: a population-based cohort study. Rheumatol Int. 2011; 31:641–645.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download