Abstract
Epithelial growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have been widely used for non-small-cell lung cancer patients. Its untoward cutaneous effects are largely well known and developed in many patients treated with EGFR TKIs. However trichomegaly of eyelash is rarely reported. Although trichomegaly is not a drug-limiting side effect, it could be troublesome of continuing the treatment because of cosmetic issue or eyeball irritation by long eyelashes. Therefore clinicians are needed to pay attention to this uncommon effect. We herein describe erlotinib induced trichomegaly of eyelashes in a woman with adenocarcinoma of the lung.
Lung cancer is the most common cause of cancer-related death in many countries. The epithelial growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have been developed and widely used in non-small-cell lung cancer (NSCLC) patients especially harboring activating EGFR mutation as first line regimen as well as second or third line treatment.
Erlotinib (Tarceva) is known to act by blocking the transmembrane EGFR and preventing its autophosphorylation. This effect is involved in cellular proliferation, apoptosis, cell cycle as well as metastatic potential. EGFR is known to be located in keratinocytes, the sweat gland apparatus, and the hair follicle1. Many patients treated with EGFR TKIs therefore developed skin toxicities such as mainly acneiform skin rash and less frequently pruritus, paronichia, skin fissures, xerosis, telangiectasias and hair changes. The skin rash is associated with favorable response to the treatment2. EGFR TKIs-associated trichomegaly of eyelash has been sporadically reported and its incidence is unknown3-7. As lung cancer physicians utilize EGFR TKIs with increasing frequency, they are needed to pay attention to various untoward effects of the drugs.
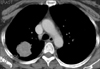
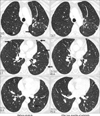
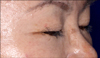
In November 2009, a 52-year-old Korean woman who had never smoked presented with a history of isolated coughing. Chest computed tomography (CT) scan revealed a 4×3 cm sized mass on the upper lobe of the left lung (Figure 1). Percutaneous needle biopsy of the lesion revealed adenocarcinoma. There was no evidence of metastases in bone scan, brain magnetic resonance imaging, or positron emission tomography (PET)-CT scan. Finally her disease was clinically staged as T2aN0M0. Polymerase chain reaction-based DNA sequencing of the EGFR gene revealed an activating mutation (Leu858Arg) in exon 21 of the EGFR gene. She underwent lobectomy of the upper lobe of the right lung. Tumor was a 4.0×4.0×3.5 cm in size and directly extended to visceral pleura on pathologic examination. She subsequently received three cycles of adjuvant chemotherapy with paclitaxel and cisplatin from December 2009. Following adjuvant chemotherapy, evaluation with chest CT scan, PET-CT scan and bone scan every three months revealed no evidence of recurrence until March 2011 when multiple small nodules were developed on both lung fields. Wedge resection of the upper lobe of the left lung was performed and metastatic adenocarcinoma was pathologically confirmed in the specimen. The DNA sequencing of the EGFR gene of resected specimen did not revealed any activating mutations of the EGFR gene. She received four cycles of chemotherapy with irinotecan and cisplatin and her disease was shown to be stable. In December 2011, pulmonary nodules were newly noted in the upper lobe of the left lung (Figure 2). Then treatment was switched to the erlotinib monotherapy, 150 mg once daily. Her disease was shown to be partial response to the treatment by criteria of Response Evaluation Criteria In Solid Tumors version 1.18 in February 2012 and has been stable until June 2012. During the treatment, she experienced skin rashes on her face, chest, and scalp, which were adequately controlled with topical therapy. In addition she was complained of excessive elongated irregular growth of both eyelashes which irritated eyeball in two months after initiation of erlotinib (Figure 3). She underwent monthly eyelash trimmings with scissors for relieving local symptom and cosmetic purpose.
The trichomegaly of eyelash is defined as excessive increase in the length, thickness, stiffness, curling, or pigmentation of eyelashes and relatively rare cosmetic disease. This was firstly reported in congenital diseases such as Oliver-McFarlane syndrome, oculocutaneous albinism type I, or familial hypertrichosis7. Acquired form of trichomegaly was also reported in certain drugs (zidovudine, latanoprost, bimatoprost, cyclosporine, topiramate, tacrolimus, and interferon-α) and some diseases (human immunodeficiency virus type 1, uveitis, dermatomyositis, and systemic lupus erythematosus).
Recently EGFR TKIs have been widely used in cancer patients, especially NSCLC. Clinicians were frequently encountered with various cutaneous effects of EGFR TKIs. Trichomegaly of eyelash as uncommon subset of side effect was reported after treatment with monoclonal antibody, cetuximab9, and EGFR TKIs such as erlotinib3,4,7 and gefitinib5,6. EGFR is located in hair follicle, especially its outer root sheath and plays an important role in regulating hair cycle1. It is plausible explanation that inhibition of EGFR signaling by these drugs may change its growth pattern and induce abnormal hair growth, trichomegaly10.
Although it is not drug-limiting side effect, patients with trichomegaly complaining of eyeball irritation are recommended to see by an ophthalmologist because of potential risk of trichomegaly induced trichiasis or corneal ulceration and differential diagnosis of other ocular diseases such as conjunctivitis and keratoconjunctivitis sicca which can be complicated with EGFR TKIs. Eyelash trimmings with scissors or epilation can be reasonable option for the patients. In this case, meibomitis and blephalitis were observed in ophthalmologic examination and treated with topical antibiotic.
In this case, activating mutation (Leu858Arg in exon 21) of the EGFR gene was noted in primary tumor while any mutation was not observed in metastatic pulmonary nodule. Her disease showed stable disease until now after partial response to erlotinib monotherapy. This discordance in mutation profile of the EGFR gene between primary and metastatic lesion could be usually observed11. Development of acneiform skin rash is known to be associated with better tumor responses to erlotinib2. However the clinical significance of trichomegaly is unknown. Prospective study is needed to verify whether trichomegaly can have predictive role or not in the treatment.
Figures and Tables
References
1. Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol. 2002. 20:110–124.
2. Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004. 22:3238–3247.
3. Carser JE, Summers YJ. Trichomegaly of the eyelashes after treatment with erlotinib in non-small cell lung cancer. J Thorac Oncol. 2006. 1:1040–1041.
4. Lane K, Goldstein SM. Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg. 2007. 23:65–66.
5. Pascual JC, Banuls J, Belinchon I, Blanes M, Massuti B. Trichomegaly following treatment with gefitinib (ZD1839). Br J Dermatol. 2004. 151:1111–1112.
6. Shah NT, Kris MG, Pao W, Tyson LB, Pizzo BM, Heinemann MH, et al. Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol. 2005. 23:165–174.
7. Braiteh F, Kurzrock R, Johnson FM. Trichomegaly of the eyelashes after lung cancer treatment with the epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol. 2008. 26:3460–3462.
8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.
9. Bouche O, Brixi-Benmansour H, Bertin A, Perceau G, Lagarde S. Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol. 2005. 16:1711–1712.
10. Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001. 144:1169–1176.
11. Daniele L, Cassoni P, Bacillo E, Cappia S, Righi L, Volante M, et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J Thorac Oncol. 2009. 4:684–688.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download