Abstract
Pigtail catheter drainage is a common procedure for the treatment of pleural effusion and pneumothorax. The most common complications of pigtail catheter insertion are pneumothorax, hemorrhage and chest pains. Cerebral air embolism is rare, but often fatal. In this paper, we report a case of cerebral air embolism in association with the insertion of a pigtail catheter for the drainage of a pleural effusion. A 67-year-old man is being presented with dyspnea, cough and right-side chest pains and was administered antibiotics for the treatment of pneumonia. The pneumonia failed to resolve and a loculated parapneumonic pleural effusion developed. A pigtail catheter was inserted in order to drain the pleural effusion, which resulted in cerebral air embolism. The patient was administered high-flow oxygen therapy and recovered without any neurologic complications.
Pigtail catheter drainage is widely used for the treatment of pleural effusion and pneumothorax since it is easier to perform and entails fewer procedures than chest tube thoracostomy1. In addition, this procedure is less traumatic and has less ambulatory limitations2. Despite these advantages, pigtail catheter drainage is associated with complications similar to tube thoracostomy. Pneumothorax, bleeding, and tube site pain are common complications; most of which are easily managed1,3.
Cerebral air embolism can be induced by pulmonary barotrauma, trauma of the chest or head and iatrogenic causes such as invasive procedures or surgery. Cerebral air embolism may result from a number of thoracic procedures such as percutaneous transthoracic needle biopsy, thoracentesis, and thoracic surgery. However, its occurrence by the pigtail catheter insertion has not been reported4,5. We experienced a case of cerebral air embolism associated with pigtail catheter insertion for drainage of a pleural effusion. Herein, we present the case report and a review of the associated literature.
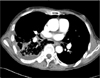
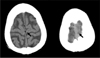
A 67-year-old man presented with dyspnea, cough and right-side chest pain that lasted for 2 days. Five years prior, he was diagnosed with chronic obstructive pulmonary disease at our hospital and had been receiving bronchodilator therapy. He was a current smoker with a 30-pack-year smoking history and was treated for pulmonary tuberculosis 2 years ago. Auscultation of the lungs revealed an inspiratory crackle at the right lower lung field. The blood pressure was 120/70 mm Hg, the pulse rate was 84 beats per minute, the respiratory rate was 18/min and the body temperature was 36.5℃. The arterial blood gas analysis showed pH 7.45, PaCO2 27.2 mm Hg, PaO2 59.0 mm Hg, HCO3 18.5 mmol/L, SaO2 92.2% at room air. The laboratory test results were white blood cells (WBC) count 16,400/mm3, hemoglobin 13.4 g/dL, platelets 194,000/mm3, and C-reactive protein 32.5 mg/dL. The chest radiograph revealed pneumonic infiltration in the right lower lobe. The chest computed tomography (CT) found emphysematous changes in the lung parenchyma and consolidation in the right lower lobe with a right pleural effusion (Figure 1). Antibiotic treatment failed to improve the right pneumonic infiltration and pleural effusion. Furthermore, a loculated fluid collection was discovered, so a pigtail catheter was inserted for pleural fluid drainage. The pigtail catheter was placed through a guide wire after thoracentesis. Pleural fluid analysis showed pH 8.0, red blood cells 460,000/mm3, WBC 1,220/mm3 (neutrophil 90%, lymphocyte 10%), protein 6.1 g/dL (plasma 7.8 g/dL), lactate dehydrogenase 176 IU/L (plasma 203 IU/L), and glucose 63 mg/dL. This finding was compatible with parapneumonic effusion, and hemothorax was suspected. As soon as the pigtail catheter was inserted, the patient complained of impaired vision. A few minutes later, he lost consciousness and had a generalized seizure. A brain CT scan was performed immediately and showed multiple low densities in the right occipital lobe, which were interpreted as gas emboli (Figure 2). Cerebral air emboli were diagnosed. High concentration oxygen inhalation therapy was initiated and he was placed in the right lateral decubitus position. About 20 minutes after the pigtail catheter insertion, the seizure stopped. However, the patient's consciousness did not improve and his respiratory rate began to decline. Auscultation was no different from the first time. The arterial blood gas analysis showed pH 7.34, PaCO2 46.2 mm Hg, PaO2 114.3 mm Hg, HCO3 20.4 mmol/L, and SaO2 99.8%. Mechanical ventilation was initiated and conservative treatment was maintained in the intensive care unit. In a chest X-ray performed after the start of mechanical ventilation, the amount of right pleural effusion had decreased and there was no pneumothorax. No air leak was found through pigtail catheter. After initiation of mechanical ventilation, diffusion-weighted image and fluid attenuated inversion recovery image of brain magnetic resonance image showed no abnormal findings, such as cerebral infarction, cerebral edema or other lesions causing the seizure after pig tail catheter insertion. Electroencephalography also did not show any epileptiform discharge. Fundoscopic examination showed no abnormal findings of the retina, such as air embolism. On the next day, his consciousness was fully recovered after conservative treatment including mechanical ventilation and high-flow oxygen therapy. His pneumonia also improved with conservative treatment. He was discharged without any neurologic complications.
Thoracentesis and chest tube insertion are procedures frequently used for the diagnosis and treatment of pulmonary and pleural diseases. Pigtail catheter drainage is performed with a small caliber catheter (less than 14 French)6. It is easier to perform, and causes less tissue injury than chest tube thoracostomy. In addition, this procedure has less ambulatory limitation, and is better-tolerated by patients than chest tube thoracostomy since no dissection of intercostal muscles and skin is involved2,3. In previous studies, pigtail catheter drainage was shown to be as effective as large bore chest tube drainage in the treatment of various pleural effusions except for pneumothorax and viscous pleural effusion6,7.
The complications of chest tube insertion include pneumothorax, hemothorax, pain, infection, dislodgement, kinking, disconnection of the chest tube, and drainage blockage8,9. Visceral organ injuries by the insertion procedure are rare, but fatal. The complications associated with the pigtail catheter drainage are similar to those of chest tube drainage, but kinking of catheter or drainage block occur more frequently because of its smaller diameter1,2.
Air embolism, the entry of gas into the venous or arterial system, may result from invasive procedures, trauma, and barotrauma of lung10. There are many reports of air embolism occurring after procedures such as percutaneous transthoracic needle biopsy or thoracentesis. However, cerebral air embolism occurring after chest tube insertion, especially the pigtail catheter insertion for drainage of pleural effusion, has not been reported.
To understand the mechanism of cerebral air embolism following pigtail catheter insertion for pleural drainage, the insertion process needs to be understood. A guidewire is inserted after thoracentesis, and the pigtail catheter is inserted through the guidewire. During this process, it is possible to puncture the lung parenchyma at any point7. The air embolism by lung puncture is thought to result from gas bubbles entering a pulmonary vein. There are several different possibilities for the mechanism of occurrence. First, air bubbles can flow in when a needle tip enters a pulmonary vein while its base is opened to the atmosphere. Once the atmospheric pressure exceeds the pulmonary venous pressure during inspiration, air enters the pulmonary venous system. Second, it may occur when a needle penetrates both an air-containing space and a nearby pulmonary vein, causing a bronchovenous fistula. Coughing or Valsalva maneuver may increase airway pressure and air enters the pulmonary vein11,12. Third, embolism may happen if air bubbles are injected into a vein in the pleura or chest wall. In this case, the air entering the venous circulation flows into the systemic arterial circulation through an intracardiac defect such as a patent foramen ovale or other mechanisms. This phenomenon is called paradoxical embolism4. For the case in this report, any one of these 3 possibilities might have been applicable.
Air bubbles entering the systemic arterial circulation can reach multiple organs and block arterioles with a diameter of 30-60 µm4. Amongst the organ injuries by air embolism, cerebral or coronary embolisms may result in fatality13.
The diagnosis of cerebral air embolism is difficult. As shown in this case, it is important to have clinical suspicion of a cerebral air embolism based on a direct relation between the neurologic symptoms and the performance of an invasive procedure. Sudden changes in sensorium are the most common symptoms and manifestations range from mild disorientation to serious symptoms such as hemiplegia, coma, and seizures5. A brain CT scan can provide a definitive diagnosis by showing air bubbles in the cerebral arteries. A fundoscopic examination may also demonstrate the air bubbles in the vasculature4,12.
If cerebral air embolism is suspected, the patient must have 100% high-flow oxygen therapy. This therapy maximizes tissue oxygenation and reduces emboli volume by eliminating nitrogen13. There are some controversies about the optimal position for prevention of cerebral air embolism when the patient is diagnosed with systemic air embolism. The right lateral decubitus position prevents air from entering the systemic arterial circulation because air bubbles remain in the non-dependent, superior aspect of the left ventricle and, away from the aorta. In contrast, the left lateral decubitus or Trendelenburg position is advocated in cases of venous air embolism4.
Hyperbaric oxygen therapy (HBOT) is the most effective treatment for air emboli. HBOT reduces the size of air emboli and improves the oxygenation of ischemic tissue by increasing the solubility of oxygen in the blood. In addition, HBOT reduces cerebral edema by decreasing the permeability of blood-brain-barrier4. Although HBOT is a relatively safe procedure, there are some absolute and relative contraindications. The absolute contraindication to HBOT is an untreated pneumothorax, as it may progress into a tension pneumothorax. The relative contraindications include chronic obstructive pulmonary disease, claustrophobia, Eustachian tube dysfunction, upper respiratory infection, seizure, and the presence of a pacemaker13. In our case, the patient did not receive HBOT because he underwent mechanical ventilation after he experienced a generalized seizure and respiratory failure. Although early application of HBOT is recommended, there are some reports that delayed application is also effective for increasing survival and neurological recovery because air bubbles have been demonstrated to exist up to 48 hours after an initial event14. Unlike many previous case reports, the patient in this case recovered without any neurologic complications even without HBOT. This may be because of the size of air embolism, which may have been too small to cause permanent neurological damage. However, if neurologic complications have not fully recovered, delayed HBOT should be considered.
In conclusion, cerebral air embolism following pigtail catheter insertion is rare, but serious and potentially fatal once it occurs. Therefore, a high level of clinical suspicion concerning the relationship between pigtail catheter insertion and neurologic symptoms is important for rapid diagnosis. Prompt management for the prevention of further air entry and conservative treatment including high-flow oxygen therapy and cardiopulmonary supportive care, are thought to be effective in mild cerebral air embolism, if HBOT is not available or contraindicated.
Figures and Tables
References
1. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006; 130:1857–1863.
2. Lin CH, Lin WC, Chang JS. Comparison of pigtail catheter with chest tube for drainage of parapneumonic effusion in children. Pediatr Neonatol. 2011; 52:337–341.
3. Gammie JS, Banks MC, Fuhrman CR, Pham SM, Griffith BP, Keenan RJ, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999; 3:57–61.
4. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000; 342:476–482.
5. Bou-Assaly W, Pernicano P, Hoeffner E. Systemic air embolism after transthoracic lung biopsy: a case report and review of literature. World J Radiol. 2010; 2:193–196.
6. Light RW. Pleural controversy: optimal chest tube size for drainage. Respirology. 2011; 16:244–248.
7. Havelock T, Teoh R, Laws D, Gleeson F. BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010; 65:Suppl 2. ii61–ii76.
8. Miller KS, Sahn SA. Chest tubes: indications, technique, management and complications. Chest. 1987; 91:258–264.
9. Poncia H, Ryan JM. An unusual complication of chest tube thoracostomy. CJEM. 2000; 2:121–123.
10. Ho AM, Ling E. Systemic air embolism after lung trauma. Anesthesiology. 1999; 90:564–575.
11. Westcott JL. Air embolism complicating percutaneous needle biopsy of the lung. Chest. 1973; 63:108–110.
12. Um SJ, Lee SK, Yang DK, Son C, Kim KN, Lee KN, et al. Four cases of a cerebral air embolism complicating a percutaneous transthoracic needle biopsy. Korean J Radiol. 2009; 10:81–84.
13. Hare SS, Gupta A, Goncalves AT, Souza CA, Matzinger F, Seely JM. Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol. 2011; 66:589–596.
14. Ohashi S, Endoh H, Honda T, Komura N, Satoh K. Cerebral air embolism complicating percutaneous thin-needle biopsy of the lung: complete neurological recovery after hyperbaric oxygen therapy. J Anesth. 2001; 15:233–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download