Abstract
We report a rare synchronous presentation of primary lung cancer and adrenal pheochromocytoma. A 59-year-old woman was diagnosed with right upper lobe non-small cell lung carcinoma measuring 2.8 cm and a right adrenal gland mass measuring 3.5 cm, which displayed increased metabolic activity on 18F-fluorodeoxyglucose positron emission tomography-computed tomography. The adrenal lesion was revealed to be asymptomatic. The patient underwent right adrenalectomy and histological examination revealed a pheochromocytoma. Ten days later, right upper lobectomy was performed for lung cancer. This case indicates that incidental adrenal lesions found in cases of resectable primary lung cancer should be investigated.
The adrenal gland is a common site of metastasis for primary lung neoplasms. In autopsies, it has been reported to occur in 18% to 42% of patients1. In cases of operable lung cancer, the frequency of isolated adrenal metastases ranges from 5% to 10% at initial presentation 2. Most adrenal masses are benign in patients with or without a known malignancy3. In cases of resectable lung cancer and unilateral adrenal mass, 60% to 74% of the lesions have been demonstrated to be benign1,3. The detection of an adrenal mass in imaging studies poses a diagnostic dilemma as to whether the lesion is a metastasis or a benign tumor. Traditionally, percutaneous needle aspiration biopsy of the adrenal lesion has been recommended in such patients to determine further therapeutic options. However, it is potentially dangerous because of procedure-related complications, such as bleeding or pain. Magnetic resonance imaging (MRI) may be a good alternative for distinguishing between benign and malignant adrenal masses4. Positron emission tomography-computed tomography (PET-CT) has also been shown to be valuable in the evaluation of adrenal masses5. However, these methods have a diagnostic limitation due to lack of pathologic information. Laparoscopy with ultrasonography is also thought to be an effective diagnostic and therapeutic option6, but due to the invasiveness of the procedure, it cannot be used in high-risk patients with underlying illness.
Herein, we report a rare case of synchronously present non-small cell lung carcinoma and pheochromocytoma, and a review of literature.
A 59-year-old woman was referred to us from the cardiology division with a radiographic abnormality on her chest X-ray. She denied any history of respiratory symptoms, including coughing, phlegm, dyspnea, chest pain, and hemoptysis. She is a current smoker who has been smoking half a pack of cigarettes per day for more than 20 years. She was diagnosed with hypertension 5 years prior and is currently on antihypertensive medications. She was otherwise healthy.
Physical examination did not reveal any specific findings in respiratory or non respiratory areas. Routine laboratory studies and blood tests were normal. Chest X-rays revealed a solitary pulmonary nodule with ground-glass opacity in the right upper lobe (Figure 1A), with an interval increase in its size compared to her 3-year-old film. A subsequent chest computed tomography (CT) scan revealed a 2.5-cm spiculated nodule with an irregular margin in the upper lobe of the lung (Figure 1B). The lesion was uncalcified with non-enhancing features, and no associated evidence of mediastinal lymphadenopathy. The right adrenal gland showed a 3.5-cm round mass, which measured 43 Hounsfield units (HU) on unenhanced and 125 HU on enhanced images (data not shown).
Fine-needle aspiration biopsy of the lung nodule revealed non-small cell lung carcinoma. For staging determination, 18F-fluorodeoxyglucose (18F-FDG) PET-CT was performed, which displayed a 2.3-cm intense hypermetabolic lesion in the right upper lobe with no metabolic evidence of lymph node metastases. In addition, focal increased metabolic activity was revealed in the right adrenal gland (Figure 2). Her blood pressure was normal (110/70 mm Hg) on admission. She had a 5-year history of hypertension, but no tachypalpitations, headache, flushing, family history of hypertension, or other endocrine disorders. Twenty-four hour urine samples demonstrated normal ranges of metanephrine (0.6 mg/day; normal range, 0-0.8 mg/day) and vanillylmandelic acid (2.5 mg/day; normal range, 0-8.0 mg/day). Biochemical tests did not reveal any abnormalities related to other functional endocrine tumors, such as Conn's syndrome or Cushing's syndrome. Her post-bronchodilator lung function test was normal: forced expiratory volume in one second (FEV1) of 1.80 L (98% of predicted value), forced vital capacity (FVC) of 2.57 L (102% of predicted value), and FEV1/FVC of 70%.
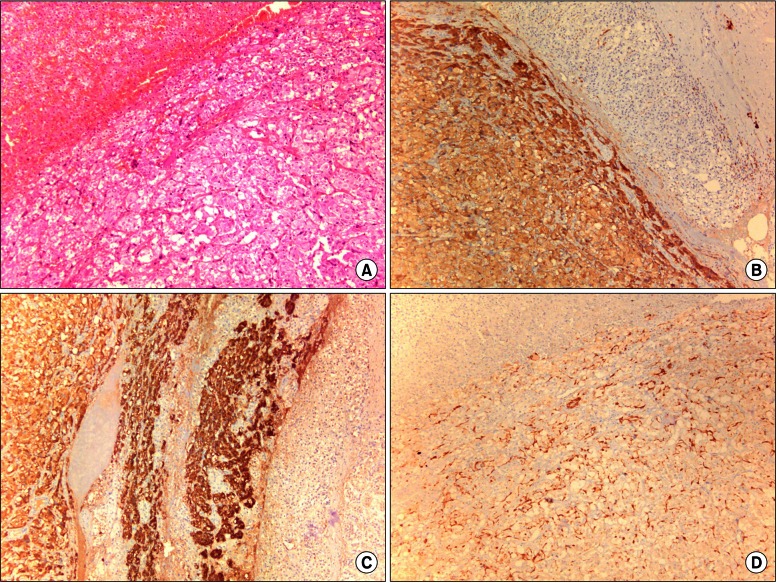
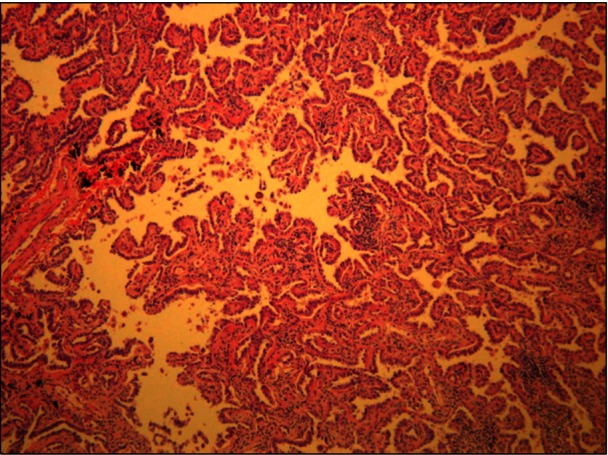
Robotic-assisted laparoscopic right adrenalectomy was performed. The adrenal gland measured 3.5×1.6 cm, weighed 17 g, and represented a pheochromocytoma, which was a well-circumscribed, reddish-brown solid 2.5-cm mass that did not invade the surrounding tissues (Figure 3). Right upper lobectomy was performed by video-assisted thoracoscopic surgery, 10 days after the adrenalectomy. The lung tumor was found to be a 2.8×2.5-cm papillary adenocarcinoma (Figure 4). The lesion was confined to the lung parenchyma with clear resection margins and no evidence of tumor involvement in the mediastinal and regional lymph nodes, consistent with stage IA disease (pT1bN0M0). Her recovery from the operation was uncomplicated. Postoperatively, she did not receive chemotherapy or radiation therapy. Two years postoperatively, there has been no tumor recurrence in the thorax and abdomen, and the patient is healthy.
Adrenal metastases from lung cancer are a common occurrence. During the course of primary lung cancer, the presence of adrenal metastases can have significant prognostic implications, because surgical resection of solitary metastases from operable lung cancer might be expected to improve survival7. Any adrenal mass found on a preoperative CT scan in a patient with lung cancer should be biopsied to exclude a benign tumor. If an adrenal metastasis is found and if the lung lesion is curable (stage I or stage II), resection of the adrenal lesion is prudent. Thus, it is very important to distinguish between a benign and a malignant lesion in patients with a known malignancy, particularly lung cancer8,9.
In this report, a 59-year-old woman was initially thought to be a case of primary lung cancer with presumptive solitary adrenal metastasis, suggesting M1b disease. Since most solitary adrenal masses are non-malignant even in patients with known lung cancer1,3,8, we thought it may be another type of adrenal disease, such as an adenoma or a functional endocrine tumor. As she had never experienced any episodes associated with catecholamine excess, we tried to perform aspiration cytology on the adrenal lesion, but the sample was unsatisfactory to make a definitive diagnosis. We did not perform further aspiration because of the possibility of significant morbidity and other complications, such as pneumothorax, infection, and hemorrhage, that have been reported in 8-13% of these patients10. Adrenal biopsy is often performed to rule out metastatic disease of the gland, but can be life-threatening as it can evoke an adrenergic storm in the case of pheochromocytoma11. Although we did not know the exact pathology of the adrenal lesion, we decided to surgically remove the lung and adrenal mass, because surgical delay in lung cancer could influence prognosis. Unfortunately, there are currently no confirmatory diagnostic modalities to distinguish benign from malignant adrenal masses. Burt et al.1 reported that the specificity of MRI in predicting a metastatic lesion was 24%, and the false-positive was 67%. More recently, Schwartz et al.9 found that in cases with lung cancer and synchronous adrenal masses, a chemical shift MRI technique showed 96% sensitivity and 100% specificity in diagnosing adrenal adenomas; therefore, it could obviate the need for needle biopsy in 55% of the cases. These data suggest that for evaluating adrenal masses, MRI may be helpful in diagnosing benign rather than malignant lesions. Metser et al.5 showed that a cutoff standardized uptake value (SUV) of 3.1 for 18F-FDG PET-CT resulted in a sensitivity of 98.5% and specificity of 92% in differentiating all adenomas from malignant lesions. The PET-CT of this patient showed increased uptake of 18F-FDG (SUV, 3.5) in the adrenal mass. Increased 18F-FDG uptake has been seen in pheochromocytoma and some adenomas, though the reason for it is not fully understood. It is suggested that the functional status of the lesions may affect the intensity of uptake, with an increased 18F-FDG uptake in functional adrenal masses12. Thus, we performed right adrenalectomy prior to right upper lobectomy.
In cases of lung cancer with a synchronous adrenal mass, the adrenal lesion could be an adenoma, metastasis, pheochromocytoma, paraganglioma, or another type of endocrine tumor. Very rarely, it could be a pheochromocytoma infiltrated with metastatic lung cancer cells13. Moreover, pheochromocytoma is a rare catecholamine-secreting neuroendocrine neoplasm originating from the chromaffin cells of the adrenal medulla with an annual incidence of about 2-8 per million people14. It is important to exclude synchronous functional adrenal tumors prior to adrenalectomy because of the rare possibility of simultaneous lung cancer and pheochromocytoma15. Biochemical testing before the biopsy of adrenal masses is also critical to exclude a catecholamine-secreting tumor because of serious potential operative complications. As the biochemical tests were within the normal ranges before surgery, the adrenal lesion of this patient is possibly a silent- or an early-stage pheochromocytoma. Additionally, her hypertension did not return to normal after the operation, and therefore, was not considered to be associated with the pheochromocytoma. She continues to be in good health, 2 years after the operations.
In conclusion, we report the successful treatment of a 59-year-old woman with a rare synchronous presentation of an early-stage non-small cell lung carcinoma and a right silent pheochromocytoma. In our opinion, the surgeries for both lung cancer and pheochromocytoma significantly improved her prognosis. This case illustrates that all synchronous adrenal masses that are displayed incidentally in cases of primary lung cancer should be investigated.
References
1. Burt M, Heelan RT, Coit D, McCormack PM, Bains MS, Martini N, et al. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. Impact of magnetic resonance imaging. J Thorac Cardiovasc Surg. 1994; 107:584–588. PMID: 8302078.
2. Porte HL, Roumilhac D, Graziana JP, Eraldi L, Cordonier C, Puech P, et al. Adrenalectomy for a solitary adrenal metastasis from lung cancer. Ann Thorac Surg. 1998; 65:331–335. PMID: 9485224.

3. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004; 25:309–340. PMID: 15082524.

4. Mitchell DG. Evaluating the impact of magnetic resonance imaging on patients with operable non-small-cell lung cancer and unilateral adrenal masses: importance of appropriate technique. J Thorac Cardiovasc Surg. 1995; 109:814–815. PMID: 7715236.

5. Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006; 47:32–37. PMID: 16391184.
6. Bendinelli C, Lucchi M, Buccianti P, Iacconi P, Angeletti CA, Miccoli P. Adrenal masses in non-small cell lung carcinoma patients: is there any role for laparoscopic procedures? J Laparoendosc Adv Surg Tech A. 1998; 8:119–124. PMID: 9681423.

7. Schuchert MJ, Luketich JD. Solitary sites of metastatic disease in non-small cell lung cancer. Curr Treat Options Oncol. 2003; 4:65–79. PMID: 12525281.

8. Oliver TW Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984; 153:217–218. PMID: 6473783.

9. Schwartz LH, Ginsberg MS, Burt ME, Brown KT, Getrajdman GI, Panicek DM. MRI as an alternative to CT-guided biopsy of adrenal masses in patients with lung cancer. Ann Thorac Surg. 1998; 65:193–197. PMID: 9456116.

10. Moreira SG Jr, Pow-Sang JM. Evaluation and management of adrenal masses. Cancer Control. 2002; 9:326–334. PMID: 12228758.

11. Sood SK, Balasubramanian SP, Harrison BJ. Percutaneous biopsy of adrenal and extra-adrenal retroperitoneal lesions: beware of catecholamine secreting tumours! Surgeon. 2007; 5:279–281. PMID: 17958227.

12. Shimizu A, Oriuchi N, Tsushima Y, Higuchi T, Aoki J, Endo K. High [18F] 2-fluoro-2-deoxy-D-glucose (FDG) uptake of adrenocortical adenoma showing subclinical Cushing's syndrome. Ann Nucl Med. 2003; 17:403–406. PMID: 12971640.

13. Mihai R, Parker AJ, Roskell D, Sadler GP. One in four patients with follicular thyroid cytology (THY3) has a thyroid carcinoma. Thyroid. 2009; 19:33–37. PMID: 18976164.

14. Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958-1981. An analysis of the National Cancer Registry Data. Acta Med Scand. 1986; 220:225–232. PMID: 3776697.
15. Chen EP, Weber CJ, Smith CD, Miller JI Jr. Synchronous presentation of primary non-small cell lung carcinoma and pheochromocytoma. Ann Thorac Surg. 2002; 74:924–926. PMID: 12238870.

Figure 1
(A) Chest radiograph revealing focal ground glass opacity in the right upper lung. (B) Chest computed tomography displaying a 2.5×1.6-cm nodule with a spiculated border containing a partial ground glass attenuated portion in the right upper lung apical segment.

Figure 2
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography scan showing intense hypermetabolic activity in the right upper lobe (A) and the right adrenal gland (B), which displayed an increased uptake of 18F-FDG (standardized uptake value, 3.5).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download