Abstract
Background
Although the lung is a common site of metastasis, endobronchial metastases (EBM) from extrathoracic malignancies are rare. Previous studies were retrospective reviews of the cases from each single institute, and the last one was performed between 1992 and 2002. We evaluated the characteristics of patients with EBM who had been diagnosed in recent 10 years in our hospital.
Methods
We retrospectively reviewed 1,275 patients who had undergone diagnostic bronchoscopic procedures between 2001 and 2011. An EBM was defined as bronchoscopically notable lesion, which was histopathologically identical to the primary tumor.
Results
A total of 18 cases of EBM were identified. The mean age was 53 years, and 12 cases of the 18 patients were female. The most common primary malignancies were colorectal cancer and breast cancer (4 cases each), followed by cervix cancer (3 cases) and renal cell carcinoma (2 cases). Cough was the most common symptom. The most common radiologic finding was atelectasis, which was identified in 27.7% of the cases. The median interval from the diagnosis of primary malignancy to the diagnosis of EBM was 14 months (range, 0-112 months). The median survival time from the diagnosis of EBM was 10 months (range, 1-39 months).
Conclusion
EBM from extrathoracic malignancies were rare. Colorectal cancer and breast cancer were common as primary malignancies. Fiberoptic bronchoscopy should be performed in all patients, who are suspected of having EBM. If atypical clinical and pathological features are present, appropriate diagnostic studies should be undertaken.
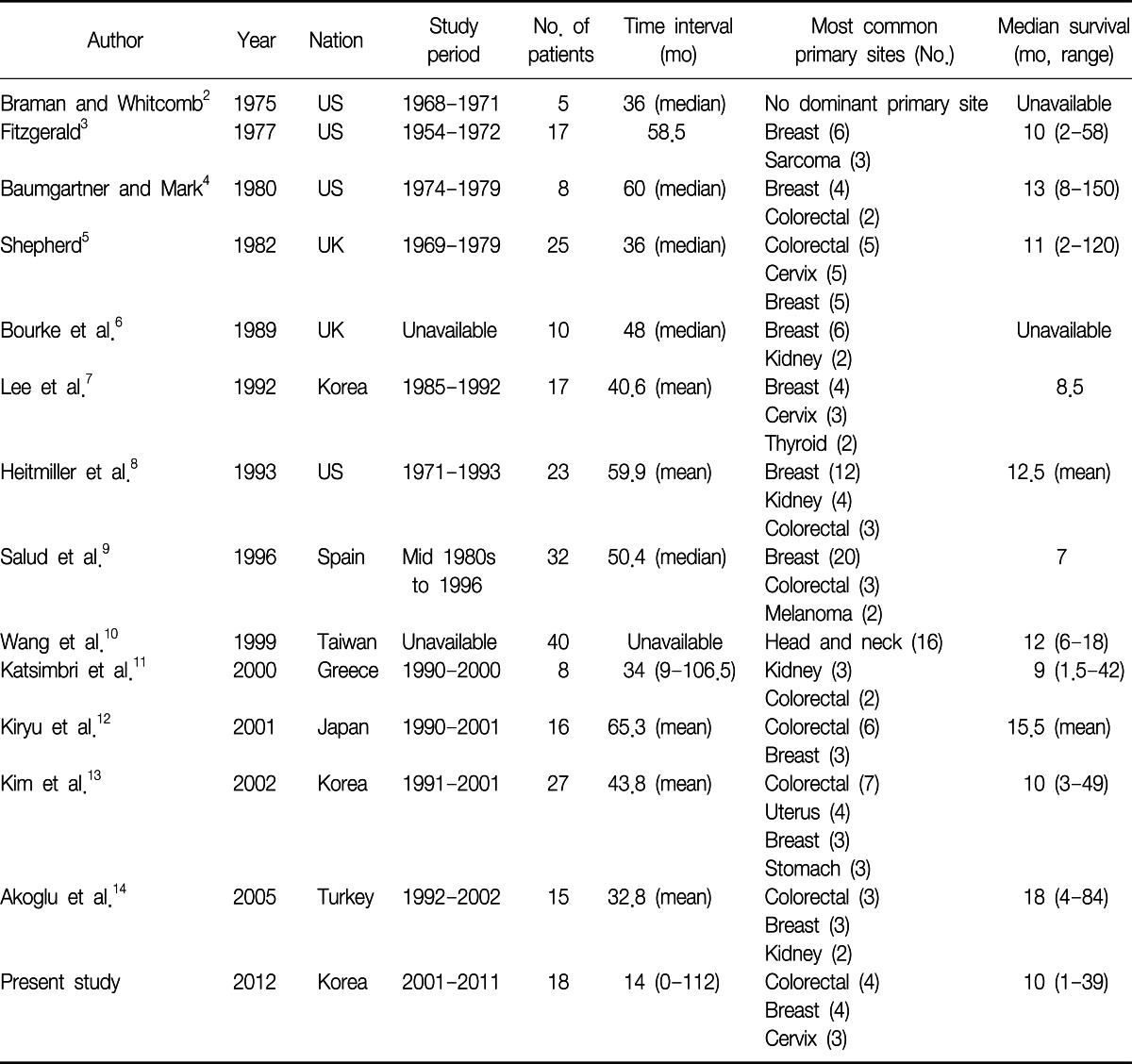
Although the lung is a common site for metastases from various extrapulmonary malignancies, endotracheal or endobronchial metastases (EBM) are rare. The frequency of EBM varies according to the definition (range, 2-28%)1,2. Rosenblatt et al.1 reported that the incidence of EBM was as high as 50% in their postmortem examination of pulmonary metastatic disease. Braman and Whitcomb2 reviewed the autopsies of patients who had died with solid tumors and reported that the incidence of EBM was as low as 2%. They counted only grossly notable endobronchial lesions. Various tumors have been associated with EBM. Treatment and management should be planned, according to the histology of the primary tumor, anatomic location, evidence of other metastasis, and the performance status of the patient. We have identified 13 studies in the literature that reviewed the prevalence and characteristics of EBM2-14. Because of low incidence of the EBM, as well as the different characteristics of each institute and the difference in time decades, the results vary from study to study. Since the last study14, which was performed between 1992 and 2002, there was no further study published in the literature. Therefore, we evaluated the clinical, radiographic and bronchoscopic aspects of patients with EBM, who had been diagnosed in recent 10 years in our hospital. We reviewed the literature and compared our cases with the previously reported series.
Between Jan 1, 2001 and Dec 31, 2011, we retrospectively reviewed the patients who had undergone diagnostic procedures using fiberoptic bronchoscopy at Bundang CHA Medical Center (Seongnam, Korea). In the study period, a total of 1,271 biopsy procedures were performed using fiberoptic bronchoscopy. The procedures were diagnostic in 641 cases. Among them, 438 cases of malignancies were identified. An EBM was defined as bronchoscopically notable lesion, which was histopathologically identical to the primary tumor. Slides and reports of biopsy of the primary tumor and endobronchial biopsy material were compared to confirm the diagnosis of EBM. We investigated the estrogen/progesterone receptor status if the primary malignancy was breast cancer, and the human papillomavirus (HPV)-DNA pattern in case of uterine cervix cancer. If the histological differentiation of the endobronchial tissue is still unclear, we compared the immunohistochemical staining, HPV-DNA pattern for cervix cancer, or estrogen/progesterone receptor status for breast cancer. Data regarding the patients' clinical characteristics, symptoms, radiographic and bronchoscopic findings were evaluated.
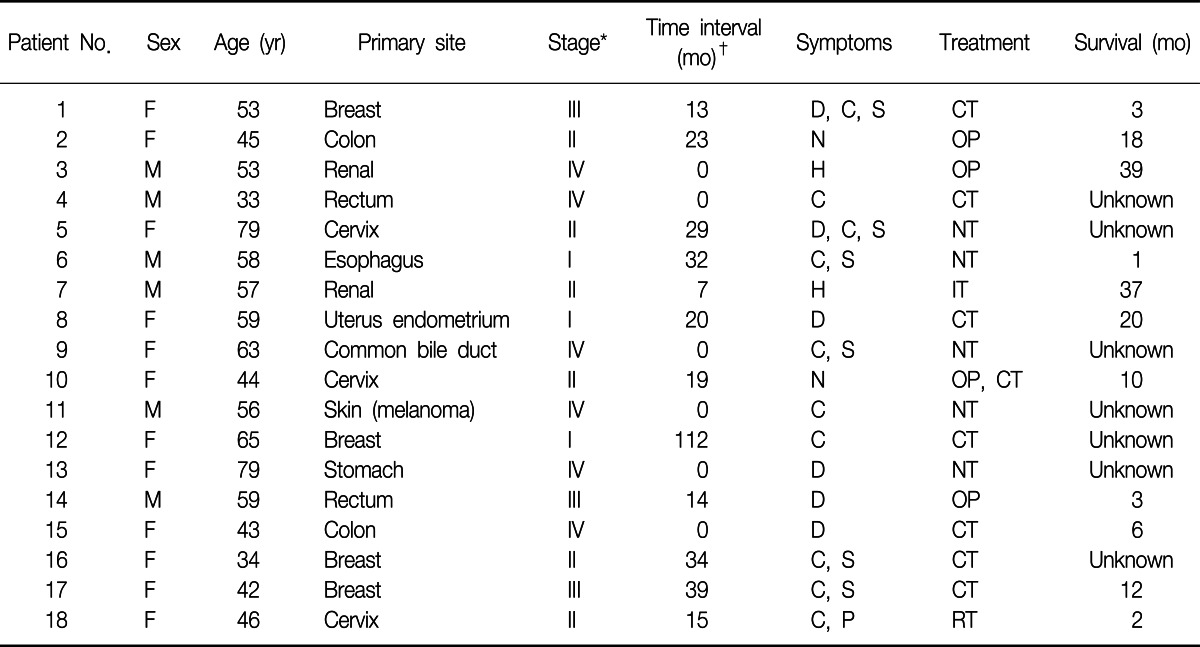
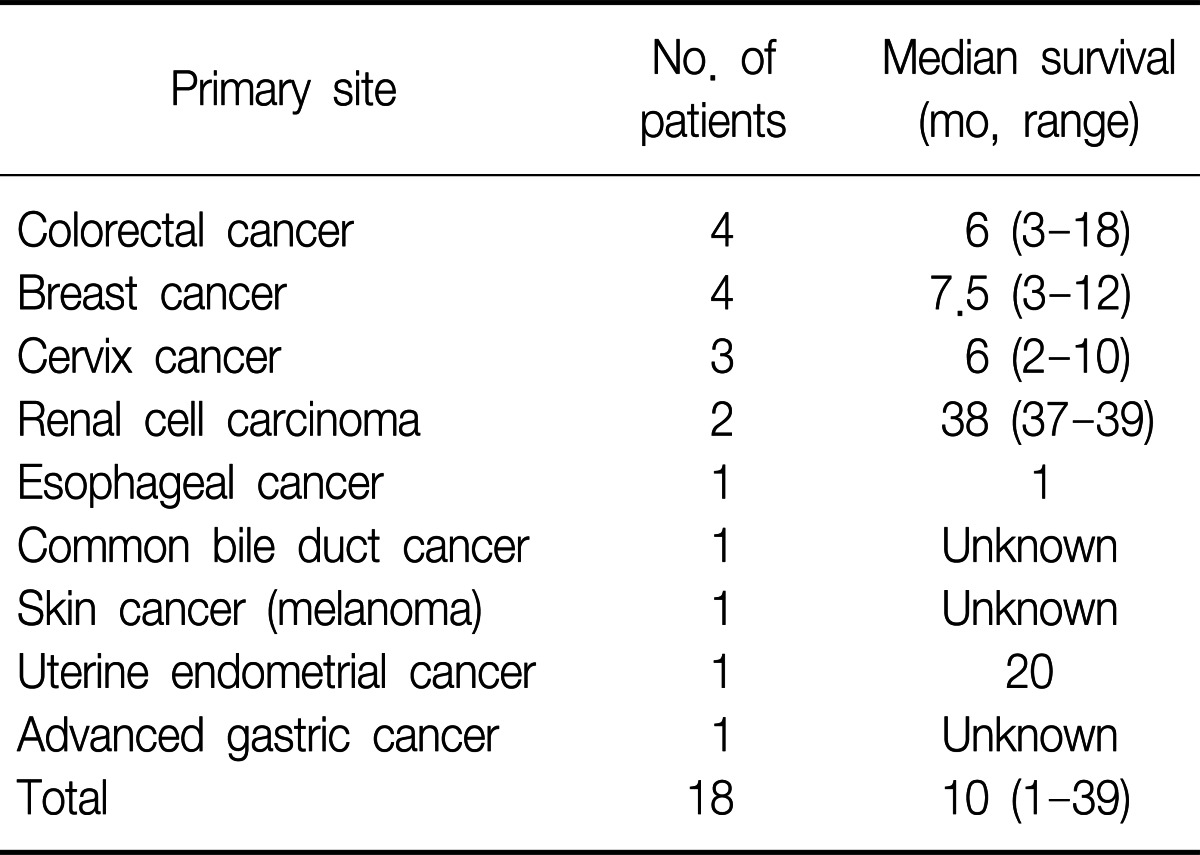
A total of eighteen patients were identified as having EBM from extrapulmonary malignancies. Among them, twelve patients were women (66.7%). The range of age was from 34 to 79 years with the median age of 53 years. The primary malignancies were 4 breast cancers, 4 colorectal cancers, 3 uterine cervix cancers, 2 renal cell carcinomas, 1 esophageal cancer, 1 cholangiocarcinoma, 1 melanoma, 1 uterine endometrial cancer, and 1 advanced gastric cancer. Table 1 shows clinical characteristics of the patients.
Cough was the most frequent symptom (10 patients, 55.6%), followed by dyspnea (6 patients, 33.3%), purulent sputum (6 patients, 33.3%), and hemoptysis (2 patients, 11.1%).
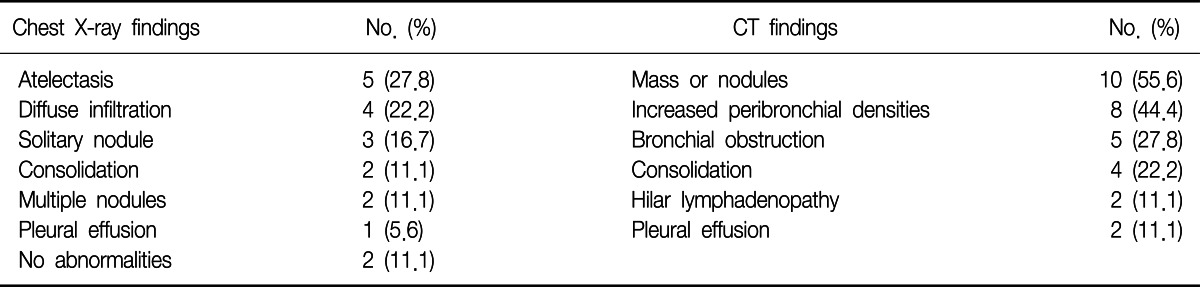
The most frequent chest radiographic findings were obstructive atelectasis (5 cases) and diffuse infiltration (4 cases), followed by solitary nodule (3 cases), consolidation (2 cases), and multiple nodules (2 cases). In two cases, chest radiograph showed no abnormalities at all. The computed tomography scan of the chest was performed on all of the patients. It revealed lung mass or nodules (10 cases), increased peribronchial densities (8 cases), bronchial obstruction (5 cases), and consolidations (4 cases) (Table 2).
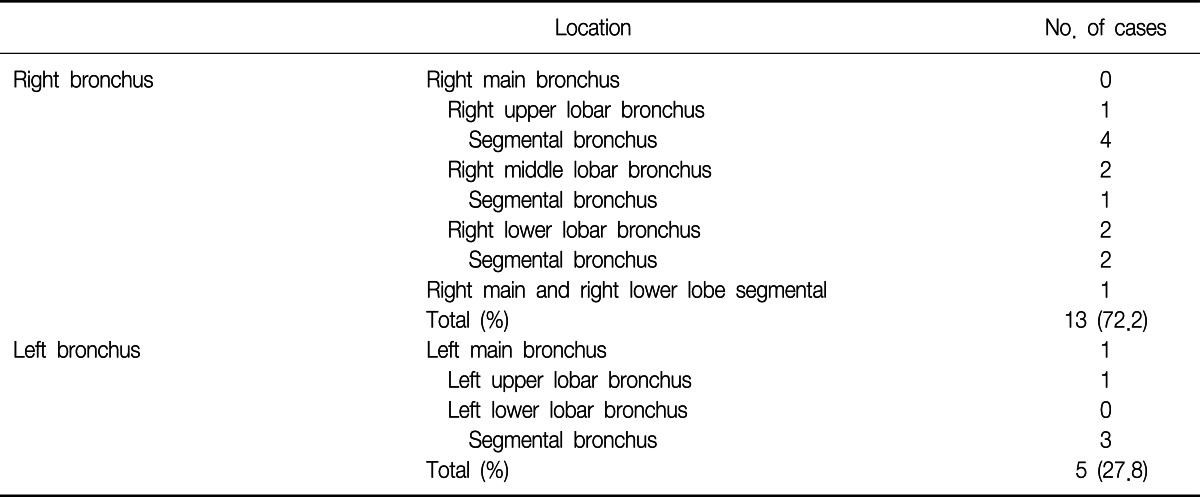
On bronchoscopic examination, all of the cases showed single lesion, except one case. The lesions were located in the segmental bronchus (10 cases), lobar bronchus (6 cases), and main bronchus (1 case). In one case, the lesions were found in multiple locations (right main bronchus and right lower lobe basal segmental bronchus) (Table 3).
The median time from the diagnosis of the primary malignancy to the diagnosis of EBM was 14.5 months (range, 0-112 months). Six patients were found to have EBM at the initial evaluation. The median time from the diagnosis of EBM to death or following loss was 3 months (range, 1-39 months). When we excluded the patients who were not followed after diagnosis, the median survival time was 10 months (range, 1-39 months) (Table 4).
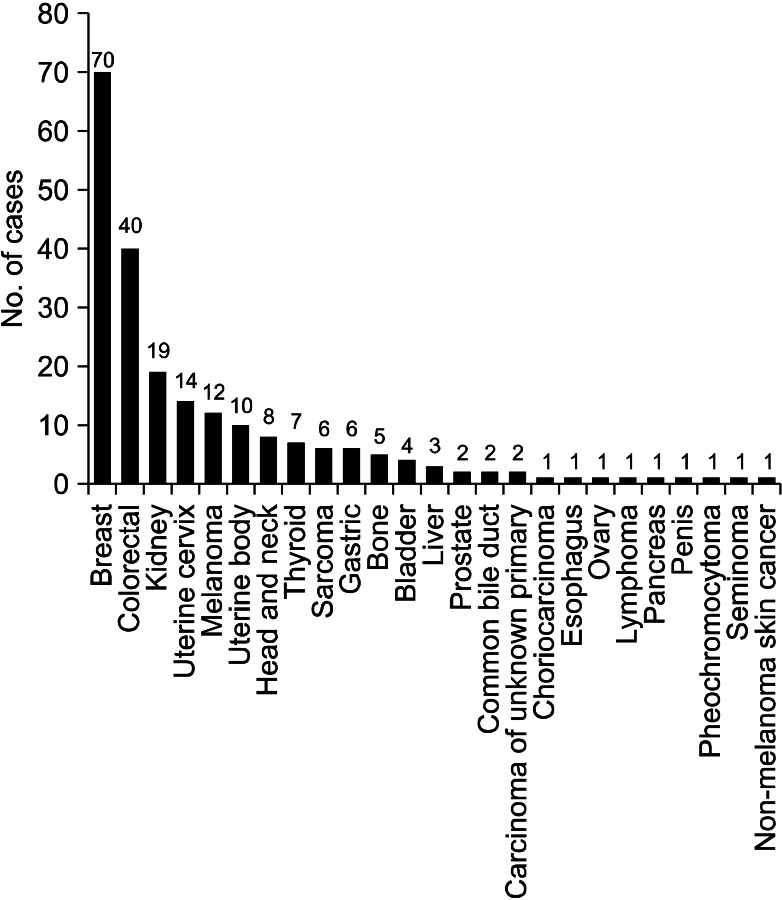
Excluding sporadic case reports of EBM, we have identified 13 single-institute studies that systematically reviewed EBM from extrapulmonary malignancies2-14 (Table 5). Among them, we could not acquire patient data of the study performed in Taiwan10, although we had tried to contact the authors. When we put all the available data together, there were a total of 219 patients and the most common primary malignancy was breast cancer, followed by colorectal cancer and renal cell carcinoma (Figure 1). Because the cancer incidence is different over decades, we divided the studies into two time periods, according to the publication year; before and after 2000.
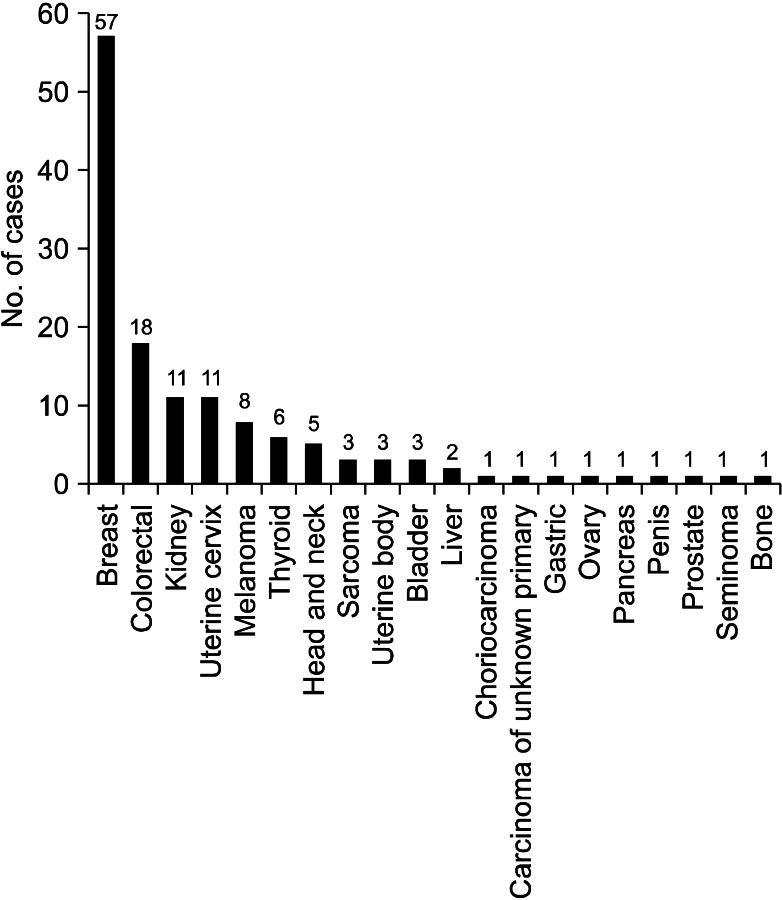
When we analyzed the studies published before 2000, the most common primary malignancy was breast cancer, followed by colorectal cancer, renal cell carcinoma, and uterine cervix cancer. There were six cases of thyroid cancer, while there was only one case of gastric cancer (Figure 2). However, in the studies published after 2000, the most common primary cancer was colorectal cancer, followed by breast cancer, renal cell carcinoma, and uterine body cancer. There were five cases of gastric cancer, while there was only one case of thyroid cancer (Figure 3).
The international trends of increasing incidence of colorectal cancer may explain the change in the proportion of colorectal cancer, between the two study periods15,16. However, we should pay attention to the difference in the ethnic background of each study. Among the eight studies published before 2000, except Taiwan study, four studies (53 patients, 39%) were performed in the Unites States, three studies (67 patients, 49%) were performed in Europe and one study (17 patients, 12%) was performed in East Asia. In contrast, among the five studies published after 2000, three studies (61 patients, 73%) were performed in East Asia, one study (15 patients, 18%) was performed in Turkey, and one study (8 patients, 9%) was performed in Greece. In the United States, the incidence of colorectal cancer is decreasing, while the international incidence of colorectal cancer is increasing15, and there is no study performed in the Unites States among the later studies. Therefore, these studies cannot be generalized to other parts of the world, which have different ethnic backgrounds. Also, the difference in the ethnic background can explain the increased proportion of gastric cancer, which is more prevalent in East Asia16, in the later studies.
The incidence of breast cancer is high in Western and Northern Europe, Australia, and North America; and low in Asia16. According to the Statistics Korea (http://www.kosis.kr/abroad/abroad_01List.jsp), the incidence of breast cancer in Korea is rising, however, the incidence of colorectal cancer is rising faster than that of breast cancer17. These trends can explain the change of proportion of breast cancer and colorectal cancer in the three Korean studies (including current one)7,13. However, the data about the incidence of cancer in Korea can't explain why gastric cancer is not the most common primary malignancy in the Korean studies. Perhaps gastric cancer can be assumed to have lower potential of metastasis to the trachea or bronchus than colorectal cancer or breast cancer. Although there has been a substantial improvement in the understatng of tumor metastasis, it is still unclear why each cancer type has different metastatic potential and metastatic sites. The anatomical relationship of the lymph-vascular systems, tumor-induced angiogenesis or lymphangiogenesis, and different chemokines may explain the discrepancies between the cancer prevalence and endobronchial metastasis18,19.
The mean time from the initial diagnosis of primary malignancy to the diagnosis of endobronchial metastasis varies from 32.8 months14 to 65.3 months12 in other studies. In this study, the median time interval of 14.5 months is relatively shorter than that of the previous reports. However, in six patients, the EBM were found at the time of the initial diagnosis of primary malignancies. When we exclude these six patients, the median time interval was 21.5 months, which is similar to the previous studies.
Among the six patients who were diagnosed primary malignancies and EBM at the same time, two patients were considered to have primary bronchogenic carcinoma at first. However, the the result of immunohistochemical staining showed unusual patterns as a primary bronchogenic carcinoma, and further diagnostic workup revealed extrapulmonary malignancies: renal cell carcinoma and cholangiocarcinoma. In most of the cases, we could make the diagnoses of EBM by comparing the histologic and immunostaining patterns. In one case, the diagnosis of EBM was made after surgical resection of the primary cancer and pulmonary metastasectomy. In three cases of breast cancers, estrogen/progesterone receptor status further helped the diagnoses. Two cases of EBM from uterine cervix cancer were further confirmed by HPV-DNA analysis.
Management of EBM should be based on the histology of the primary tumor, anatomic location of EBM, other metastatic sites, and the performance status of the patients. In our series, 8 patients were treated with chemotherapy agents. If there was no other metastatic lesion and the primary lesion was expected to be controllable, surgical resection was performed (in 4 patients). The median survival of the patients who underwent surgical resection was 14 months (range, 3-39 months). Among them, one patient (cervix cancer) died from disease progression 10 months after surgical resection. A patient with colon cancer died from pneumonia 3 months after surgical resection. Other two patients (colon cancer and renal cell carcinoma) showed fairly long-term survival of 18 and 39 months, respectively. However, we could not analyze the benefit of surgical resection because the patient number was too small. Interventional bronchoscopy, such as neodymium doped-yttrium aluminum garnet (Nd-Yag) laser debulking or intraluminal radiotherapy can be used for palliation of symptoms20-23. The median survival from the diagnosis of EBM was 10 months in our study, and this is also similar to the survival data of the previous studies (range, 7-18 months)9,14. The reason why renal cell carcinoma showed relatively long term survival compared to the other cancers in our study is unclear. Generally, the survival is dependent to a great degree on biologic behavior of the particular tumor and its responsiveness to the treatments. EBM is generally a manifestation of far advanced disease state. Therefore, survival after the diagnosis of EBM is poor.
In about 72% of the cases, the lesions were found in the right bronchial tree in our study. Lee et al.7 reported 56% of EBM were found in the right bronchial tree. Heitmiller et al.8 also reported 54%, Salud et al.9 reported 56%, Kiryu et al.12 reported 80%, Kim et al.13 reported 70%, and Akoglu et al.14 reported 56.5% of the lesions were found in the right bronchus. It is unclear why endobronchial metastasis is more frequently found in the right bronchial tree.
Cough was the most common symptom in our series (10 cases, 56%), followed by dyspnea (6 cases, 33%) and sputum (6 cases, 33%). Although there were some variations, cough and dyspnea were the most common symptoms in other studies8,9,12-14. Radiologic findings of this study were also similar to the results of the previous studies.
In conclusion, EBM from extrathoracic malignancies were rare. Reviewing a total of 18 cases of EBM of extrathoracic malignancies, we found colorectal cancer and breast cancer were the most common primary malignancies, followed by uterine cervix cancer. The cases we have reported are similar to those found in the previous studies, regarding their symptoms, radiological findings, and clinical outcomes. Fiberoptic bronchoscopy should be performed in all patients with extrapulmonary tumor, who are suspected to have endobronchial metastasis to differentiate it from the primary lung cancer. If atypical clinical features are present or atypical cell type is discovered by bronchoscopy, appropriate diagnostic studies should be undertaken.
References
1. Rosenblatt MB, Lisa JR, Trinidad S. Pitfalls in the clinical histologic diagnosis of bronchogenic carcinoma. Dis Chest. 1966; 49:396–404. PMID: 5931036.
2. Braman SS, Whitcomb ME. Endobronchial metastasis. Arch Intern Med. 1975; 135:543–547. PMID: 1138668.

4. Baumgartner WA, Mark JB. Metastatic malignancies from distant sites to the tracheobronchial tree. J Thorac Cardiovasc Surg. 1980; 79:499–503. PMID: 7359928.

6. Bourke SJ, Henderson AF, Stevenson RD, Banham SW. Endobronchial metastases simulating primary carcinoma of the lung. Respir Med. 1989; 83:151–152. PMID: 2602599.

7. Lee HL, Kwak SM, Chang JH, Kim SK, Kim SK, Lee WY, et al. Endobronchial metastasis of extrapulmonary malignancies. Korean J Intern Med. 1992; 43:806–813.
8. Heitmiller RF, Marasco WJ, Hruban RH, Marsh BR. Endobronchial metastasis. J Thorac Cardiovasc Surg. 1993; 106:537–542. PMID: 8361198.

9. Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996; 62:249–252. PMID: 8691837.

10. Wang YH, Wong SL, Lai YF, Lin AS, Chang HW. Endobronchial metastatic disease. Changgeng Yi Xue Za Zhi. 1999; 22:240–245. PMID: 10493029.
11. Katsimbri PP, Bamias AT, Froudarakis ME, Peponis IA, Constantopoulos SH, Pavlidis NA. Endobronchial metastases secondary to solid tumors: report of eight cases and review of the literature. Lung Cancer. 2000; 28:163–170. PMID: 10717334.

12. Kiryu T, Hoshi H, Matsui E, Iwata H, Kokubo M, Shimokawa K, et al. Endotracheal/endobronchial metastases: clinicopathologic study with special reference to developmental modes. Chest. 2001; 119:768–775. PMID: 11243955.
13. Kim DH, Park MS, Chung JH, Cheong JH, Kim SK, Chang J, et al. Endobronchial metastasis of extrapulmonary malignancies. Tuberc Respir Dis. 2002; 53:285–293.

14. Akoglu S, Ucan ES, Celik G, Sener G, Sevinc C, Kilinc O, et al. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis. 2005; 22:587–591. PMID: 16475029.

15. Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009; 18:1688–1694. PMID: 19505900.

16. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.

17. Statistics Korea [Internet]. c1999-2009. cited 2012 Sep 12. Daejeon: Korean Statistical Information Service;Available from: http://www.kosis.kr/abroad/abroad_01List.jsp.
18. Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009; 125:2747–2756. PMID: 19569051.

19. Sleeman JP, Cady B, Pantel K. The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clin Exp Metastasis. 2012; 29:737–746. PMID: 22669542.

20. Carlin BW, Harrell JH 2nd, Olson LK, Moser KM. Endobronchial metastases due to colorectal carcinoma. Chest. 1989; 96:1110–1114. PMID: 2805842.

21. Pisch J, Villamena PC, Harvey JC, Rosenblatt E, Mishra S, Beattie EJ. High dose-rate endobronchial irradiation in malignant airway obstruction. Chest. 1993; 104:721–725. PMID: 7689945.

22. Quantrill SJ, Burt PA, Barber PV, Stout R. Treatment of endobronchial metastases with intraluminal radiotherapy. Respir Med. 2000; 94:369–372. PMID: 10845436.

23. Solomonov A, Rosenblatt E, Ben-Izhak O, Goralnik L, Yigla M. High-dose-rate endobronchial brachytherapy in endobronchial metastatic malignant chondroid syringoma. Respiration. 2001; 68:406–410. PMID: 11464090.

Figure 3
Primary malignancies from the results of previous studies that were published after 2000 including ours.
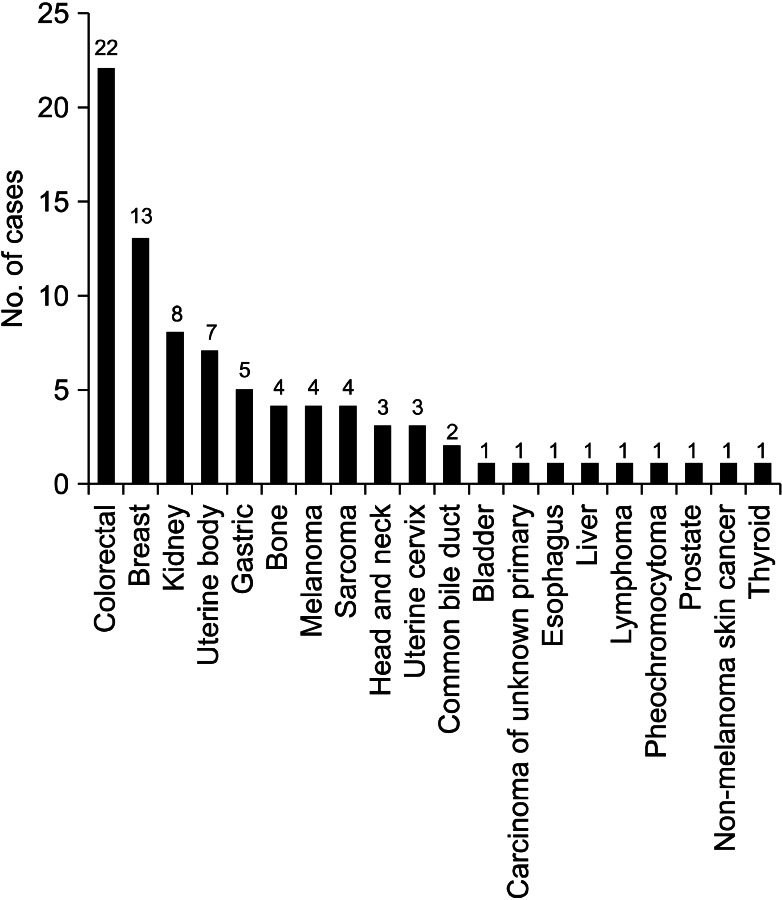
Table 1
Patients characteristics
*TNM stage at initial diagnosis of primary malignancy. †Time interval from initial diagnosis to endobronchial metastasis.
F: female; M: male; D: dyspnea; C: cough; S: sputum; N: no symptom; H: hemoptysis; P: pain; CT: chemotherapy; OP: operation; NT: no treatment; IT: immunotherapy; RT: radiotherapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download