Abstract
Background
Differentiating cardiogenic pulmonary edema from other bilateral lung diseases such as pneumonia is frequently difficult. We conducted a retrospective study to identify predictors for cardiogenic pulmonary edema and non-cardiogenic causes of bilateral lung infiltrates in chest radiographs.
Methods
The study included patients who had newly developed bilateral lung infiltrates in chest radiographs and patients who underwent echocardiography. Cases were divided into two groups based on the echocardiographic findings: the cardiogenic pulmonary edema group and the non-cardiogenic group. Clinical characteristics and basic laboratory findings were analyzed to identify predictors for differential diagnosis between cardiogenic and non-cardiogenic causes of bilateral chest infiltrates.
Results
We analyzed 110 subjects. Predictors of cardiogenic pulmonary edema were higher brain natriuretic peptide (BNP) levels, lower C-reactive protein (CRP) levels on the day of the event (<7 mg/dL), age over 60 years, history of heart disease, and absence of fever and sputum. CRP on the day of the event was an independent factor to differentiate cardiogenic and non-cardiogenic causes of newly developed bilateral chest infiltrates. Also, the validity was comparable to BNP.
Many patients visit the emergency department of primary care centers with acute shortness of breath, and their chest radiographs show bilateral haziness. Many kinds of diseases can cause bilateral chest infiltrates and can be classified as cardiogenic pulmonary edema or non-cardiogenic-related, with pneumonia as one of the most important non-cardiogenic causes. Pneumonia should be treated with proper antibiotics and hydration, whereas pulmonary edema should be treated with diuretics. Thus, a differential diagnosis should be made promptly to adequately manage the patient.
Plasma concentrations of certain natriuretic peptides or their precursors, especially brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide, are helpful in the diagnosis of heart failure. Several clinical and epidemiological studies have demonstrated a direct relationship between increasing plasma concentrations of natriuretic peptides and decreasing cardiac (usually left ventricular [LV]) function1-3. A large study has confirmed that BNP levels can help in differentiating cardiac failure from respiratory acute breathlessness in the US emergency room setting4. Monitoring BNP levels also improves the care of patients with acute dyspnea and thereby reduces hospital time and total treatment cost5.
C-reactive protein (CRP) is an acute-phase reactant that is synthesized by hepatocytes. Its production is stimulated mainly by interleukin-6, interleukin-1β, and tumor necrosis factor-β in response to infection or tissue inflammation6. Since its identification in 1930, CRP levels have been studied as a screening device for inflammation, a marker for disease activity, and a diagnostic adjunct7. Measurement of CRP levels is regarded as the most suitable tool in the differential diagnosis of pneumonia and other diseases8-10, and in general practice, can be used to identify infection due to the rapid test time and cost-effectiveness.
However, based on emerging data, CRP levels can be also used as an index for systemic inflammation in acute decompensated heart failure11 and as an independent predictor for long-term outcome in heart failure12. Data showed that CRP levels could be elevated higher than 10 mg/dL under noninfectious conditions of patients with heart failure. Patients with heart failure and LV systolic dysfunction had higher CRP levels (10.9±18.0 mg/dL) than those with no heart failure (7.9±12.1 mg/dL, p<0.001)13. In another study, CRP levels significantly increased in patients with acute pulmonary edema compared to patients with stable heart failure who were completely asymptomatic and had never sustained an episode of acute congestive heart failure14. Thus, CRP may not be suitable as an index for differential diagnosis of cardiogenic and non-cardiogenic cases, especially pneumonia. So, we conducted a retrospective study to identify predictors for cardiogenic and non-cardiogenic causes of acute bilateral lung infiltrates in chest radiographs and to elucidate whether CRP levels compared to BNP levels aid in differential diagnosis.
Our study was designed as a retrospective study at Seoul National University Hospital, which is a tertiary referral center. From September 1, 2008 to May 31, 2009, we included patients who had newly developed bilateral lung infiltrates in chest radiographs and underwent echocardiography within 48 hours from an emergency department visit.
Exclusion criteria were concomitant cardiogenic pulmonary edema and pneumonia, some combined other infection excluding pneumonia, neutropenia, a known malignancy with lung metastasis, and primary lung cancer.
Using echocardiographic findings, we divided patients into two groups, the cardiogenic pulmonary edema group and the non-cardiogenic group. Patients with LV systolic dysfunction or those with LV diastolic dysfunction were placed in the cardiogenic pulmonary edema group (i.e., the cardiogenic group) and patients lacking these features were placed in the non-cardiogenic group. Systolic dysfunction was defined as LV ejection fraction (LVEF) below 45% (LVEF≤45%)15, and diastolic dysfunction (heart failure with preserved ejection fraction) was defined as preserved LVEF (>45%) with LV relaxation abnormality or diastolic stiffness (E/E'ratio>15)16.
Age, gender, and past medical histories were reviewed from medical records. Clinical characteristics including vital signs and basic laboratory findings including CRP and BNP levels were analyzed.
To find patients with pneumonia in the non-cardiogenic group, we used following clinical criteria which were the presence of a new or progressive infiltrate in the chest radiograph, plus at least 2 of the following17: acute respiratory symptoms or changes in chronic symptom characteristics, fever above 38℃ during the initial 24 hours of hospital admission, leukocyte count>12.0 (×103/µL), and microbiological confirmation with isolated microorganisms known to be pulmonary pathogens. These criteria were also used to exclude patients with pneumonia in the cardiogenic group.
To compare and assess the extent of radiologic infiltration, we used the lung scoring system18. Lung fields in the chest X-ray were divided into three zones by imaginary horizontal lines and further divided into medial and lateral halves using an imaginary vertical line between the midline (vertebral spines) and the vertical line along the outer margin of the rib cages. Thus, each lung was divided into six segments. Based on the radiolucency of the lung infiltrates in the chest X-ray, scoring points (0 to 2; 0, normal alveolar picture; 1, patchy white infiltrates; 2, totally white infiltrates) were assigned to each segment18. Lung injury scores were not statistically different between the two groups.
This research was approved by the Institutional Review Board of Seoul National University, and has therefore been performed in accordance with the Declaration of Helsinki.
We first conducted a univariable analysis to compare clinical characteristics between the cardiogenic and the non-cardiogenic group using student's t-test and Mann-Whitney U-test for continuous variables, and the chi-square test and Fisher's exact test for categorical variables. We then made statistical models for multivariable analyses. Variables with p<0.20 in univariable analyses were included in the initial multivariable models, and the backward elimination method with a likelihood ratio test was used to obtain a final model. We made two models: the CRP-model in which BNP levels were not included and the BNP-included model. The two final models were compared in classification power using Stat's roccomp command. The cutoff value of sensitivity and specificity of two models was based on the median value of the BNP and CRP levels. p<0.05 were regarded as statistically significant, and all statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and STATA version 11.1 (Statacorp, College Station, TX, USA).
Among patients who visited the emergency department during the study period, 250 patients showed bilateral chest infiltrates and underwent echocardiography within 48 hours of the initial chest X-ray. In total, 140 patients were excluded before data analysis, and 110 patients met the inclusion criteria for the study. Exclusion criteria were other infections except pneumonia (n=50), heart failure combined with pneumonia (n=45), malignancy with lung metastasis or primary lung cancer (n=27), and neutropenia (n=18).
Characteristics of the two patient groups are given in Table 1. The cardiogenic group had 60 patients (54.5%) and the non-cardiogenic group had 50 (45.5%). Based on previously mentioned criteria, 40 patients (80%) of the non-cardiogenic group were diagnosed with pneumonia. Smokers were more prevalent in the cardiogenic group with statistical significance. The proportions of patients with dyslipidemia and heart disease (acute coronary syndrome, coronary artery bypass grafting, valvular heart disease, and arrhythmia) were higher in the cardiogenic group, and more patients with malignancy were in the non-cardiogenic group.
In the univariable analysis, cough, sputum, fever, and chilling sensations were more prevalent in the non-cardiogenic group, and body temperature was also higher in that group. Systolic and diastolic blood pressures were significantly higher in the cardiogenic group. The percentage of segmented neutrophils and fibrinogen levels were higher in the non-cardiogenic group. Higher albumin and BNP levels were observed in the cardiogenic group. CRP values on the day of the event and the following day were significantly higher in the non-cardiogenic group than the cardiogenic group, but no difference was observed over time (Table 2, Figure 1).
Echocardiographic findings of the two groups showed significant differences in LV dimension and LVEF (Table 3).
Table 4 shows the final model without BNP levels as covariates. CRP levels greater than 7 mg/dL on the day of the event showed a 5.6 times higher possibility of non-cardiogenic causes even when other covariates were adjusted. Other independent predictors of non-cardiogenic causes were no history of heart disease and the presence of sputum and fever.
We performed a multivariable analysis including BNP levels as covariates (Table 5). An age over 60, presence of dyslipidemia, and higher BNP levels were independent predictors for the cardiogenic group, whereas the presence of sputum, fever, and chilling sensations were predictors for the non-cardiogenic group. In the BNP-included model, CRP levels did not remain as independent predictors.
Two models showed good calibration and discrimination (Figure 2). No significant difference in discrimination power was detected between the CRP- and BNP-models (p=0.535). The CRP-model showed a sensitivity and specificity of 82.0% and 90.0%, respectively.
This study showed that CRP levels above 7 mg/dL on the day of the event showed an over fivefold higher possibility of non-cardiogenic causes even when other covariates were adjusted. In addition, CRP levels were comparable to BNP levels as a clinical predictor for the differential diagnosis of cardiogenic and non-cardiogenic causes in newly developed bilateral infiltrative chest lesions.
Acute shortness of breath with bilateral chest infiltrates in a simple chest radiograph is a common emergency department presentation and can be associated with a long list of differential diagnoses. Differentiating between cardiogenic causes, such as pulmonary edema with heart failure, and non-cardiogenic causes, including pneumonia, which need specific evaluation and management, is most important. Echocardiography can be used to assist in the diagnosis of heart failure, but limited availability in the acute setting and high cost frequently limit its use. Therefore, other simple and useful screening tests to predict cardiogenic causes are needed.
BNP is a natriuretic peptide produced within the heart and is released in response to ventricular overload and stretching. Several studies2,19,20 have demonstrated a marked elevation in BNP plasma levels in patients with systolic failure of the left ventricle. Furthermore, studies have shown that a restrictive filling pattern associated with systolic dysfunction is predictive of even higher BNP levels21,22. This convenient tool for the evaluation of dyspnea, especially from cardiogenic and non-cardiogenic (respiratory) causes, is widely used in clinical settings in conjunction with other clinical information. However, the high cost of the BNP test is a limiting factor in screening for acute dyspnea.
Many studies have established a relationship between CRP levels and lower respiratory tract infection2,19,20. They provided evidence for the usefulness of CRP levels to diagnose pneumonia and to assess the severity of pneumonia, which suggests the utility of CRP levels as a screening tool for the differentiation of bilateral infiltrates. However, several studies have reported increases in CRP levels in patients with chronic heart failure as a marker of systemic inflammation and as a prognostic indicator of heart failure11,12,14. Increased CRP levels have also been observed in acute decompensated heart failure of cardiogenic pulmonary edema patients, although considerable variation was seen in the CRP values (range, 2.6-25 mg/dL) according to various studies11-13. Thus, whether CRP levels can be a useful predictor in differentiating between cardiogenic and non-cardiogenic causes has been unclear.
This study showed that CRP levels were more elevated in the non-cardiogenic group than the cardiogenic group, and 80% of the non-cardiogenic group had pneumonia. CRP levels above 7 mg/dL (median value) at the day of the event showed a 5.6-fold higher possibility of non-cardiogenic causes. Moreover, in the comparison between the CRP and BNP models, no significant difference was observed in sensitivity, specificity, and area under the receiver operating curve, which suggests comparable predicting power between the CRP and BNP models. CRP models with a relatively lower cost than BNP models can be effectively used to differential diagnose acute dyspnea from bilateral infiltrates.
In the non-cardiogenic group of our study, CRP levels peaked at day 2 and gradually decreased to lower than day 1 CRP levels. However, in the cardiogenic group, CRP levels peaked at day 3 and slowly decreased, but never reached below the initial value. The last measurement of CRP values was higher than day 1 in the cardiogenic group. The change in CRP levels of the non-cardiogenic group was much more variable and faster than that of the cardiogenic group whose CRP levels were less variable and slower.
This observation can be explained by differences in the CRP kinetics between the two groups. In heart failure, baseline CRP levels were slightly elevated due to systemic inflammation and were stimulated in a limited degree due to an episode of acute exacerbation23. In pneumonia, the basal level is low and an infection stimulates rigorous CRP production, with CRP levels reaching a steady state by 48 hours and eventually decreasing once the stimulus has been removed23.
Delays in properly managing the cardiogenic group may explain the aforementioned phenomenon. Initially, antibiotics were used in 40% of the cardiogenic group (data not shown). For final outcomes, such malpractice wastes resources and needs to be reduced. However, these findings demonstrate the difficulty in differential diagnosis in cases of bilateral chest infiltrates.
This study has several limitations. Because it was performed retrospectively, the usable variables were limited. Some physical findings such as jugular venous pressure and bilateral breathing sounds, which are important in diagnosing cardiogenic pulmonary edema, were not available due to limited records.
We also did not include patients with lung cancer or metastatic lesions of the lung. Our study targeted patients with newly developed bilateral chest infiltrates. Determining whether the chest lesions of patients with lung cancer or metastatic lesions were new or old was difficult, so those cases were excluded.
We did not include patients with both cardiogenic pulmonary edema and pneumonia concomitantly. Because, our study aimed that if there are good predictors for cardiogenic vs. non-cardiogenic causes in bilateral chest infiltrates. To achieve this objective, the gray zone patients between the cardiogenic and non-cardiogenic groups should be excluded. However, in the actual clinical setting, many patients may fall in that zone, making it difficult to solve with clinical research.
In summary, higher BNP levels, lower CRP levels at the day of the event (especially below 7 mg/dL), a history of heart disease, and absence of fever and sputum were predictors of cardiogenic pulmonary edema. CRP levels on the day of the event comprised an independent factor in differentiating between cardiogenic and non-cardiogenic causes of newly developed bilateral chest infiltrates. Also, the validity was comparable to that of BNP.
This study suggests that when you meet a patient with acute bilateral lung infiltrates in chest radiographs, clinical symptoms (sputum and fever), a medical history (dyslipidemia and heart disease), and laboratory findings (BNP and CRP) may be helpful in the differential diagnosis of cardiogenic pulmonary edema and non-cardiogenic causes such as bilateral pneumonia.
Figures and Tables
 | Figure 1Changes in C-reactive protein (CRP) values over days between the two groups. CRP values (mg/dL) of the cardiogenic group are expressed by the solid line and those of the non-cardiogenic group by the dotted line. *p<0.05. |
 | Figure 2Comparison of the C-reactive protein (CRP) model and brain natriuretic peptide (BNP) model for predicting cardiogenic diseases. Filled circles represent the CRP model and open diamonds the BNP model. No significant difference was detected in discrimination power (area under the curve, 0.909 vs. 0.986; p=0.535). |
Table 3
Echocardiographic findings of the two groups
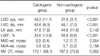
Values are presented as mean (±standard deviation).
LVID sys: left ventricular internal dimension, systolic; LVID dia: left ventricular internal dimension, diastolic; LA: left atrium; LVEF: left ventricular ejection fraction; MVE-vel: peak early diastolic mitral inflow velocity; MVA-vel: peak late diastolic mitral inflow velocity or atrial kick; MV DT: mitral valve deceleration time.
References
1. Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet. 1993. 341:1105–1109.
2. McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998. 351:9–13.
3. Groenning BA, Nilsson JC, Sondergaard L, Kjaer A, Larsson HB, Hildebrandt PR. Evaluation of impaired left ventricular ejection fraction and increased dimensions by multiple neurohumoral plasma concentrations. Eur J Heart Fail. 2001. 3:699–708.
4. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002. 347:161–167.
5. Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004. 350:647–654.
6. Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990. 12:1179–1186.
7. Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999. 17:1019–1025.
8. Almirall J, Bolibar I, Toran P, Pera G, Boquet X, Balanzo X, et al. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest. 2004. 125:1335–1342.
9. Stolz D, Stulz A, Muller B, Gratwohl A, Tamm M. BAL neutrophils, serum procalcitonin, and C-reactive protein to predict bacterial infection in the immunocompromised host. Chest. 2007. 132:504–514.
10. Castro-Guardiola A, Armengou-Arxe A, Viejo-Rodriguez A, Penarroja-Matutano G, Garcia-Bragado F. Differential diagnosis between community-acquired pneumonia and non-pneumonia diseases of the chest in the emergency ward. Eur J Intern Med. 2000. 11:334–339.
11. Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, et al. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999. 22:811–813.
12. Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca HP, Perruchoud AP. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006. 151:845–850.
13. Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007. 153:1048–1055.
14. Milo O, Cotter G, Kaluski E, Brill A, Blatt A, Krakover R, et al. Comparison of inflammatory and neurohormonal activation in cardiogenic pulmonary edema secondary to ischemic versus nonischemic causes. Am J Cardiol. 2003. 92:222–226.
15. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005. 26:1115–1140.
16. Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009. 53:905–918.
17. Campbell GD. Overview of community-acquired pneumonia: prognosis and clinical features. Med Clin North Am. 1994. 78:1035–1048.
18. Tripathi M, Pandey M, Nepal B, Rai H, Bhattarai B. Evaluation of lung infiltration score to predict postural hypoxemia in ventilated acute respiratory distress syndrome patients and the lateralization of skin pressure sore. Indian J Med Sci. 2009. 63:392–401.
19. Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997. 350:1349–1353.
20. Davis M, Espiner E, Richards G, Billings J, Town I, Neill A, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet. 1994. 343:440–444.
21. Yu CM, Sanderson JE, Shum IO, Chan S, Yeung LY, Hung YT, et al. Diastolic dysfunction and natriuretic peptides in systolic heart failure: higher ANP and BNP levels are associated with the restrictive filling pattern. Eur Heart J. 1996. 17:1694–1702.
22. Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998. 135(5 Pt 1):825–832.
23. Joffe E, Justo D, Mashav N, Swartzon M, Gur H, Berliner S, et al. C-reactive protein to distinguish pneumonia from acute decompensated heart failure. Clin Biochem. 2009. 42:1628–1634.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download