Abstract
Pleural effusion is a rare complication in non-tuberculous mycobacterial infection. We report a case of Mycobacterium intracellulare pleuritis with idiopathic pulmonary fibrosis in a 69-year-old man presenting with dyspnea. Pleural effusion revealed lymphocyte dominant exudate. M. intracellulare was identified using a polymerase chain reaction-restriction fragment length polymorphism method and liquid cultures of pleural effusion and pleural biopsy. After combination therapy for M. intracellulare pulmonary disease, the patient was clinically well at a 1-month follow-up.
Pulmonary disease is one of the most common manifestations of non-tuberculous mycobacteria (NTM) infections, and Mycobacterium intracellulare is the most common causative organism1-9. NTM are a group of over 100 species of bacteria that are ubiquitous in soil and water. NTM are opportunists, requiring defects in local or systemic host immunity in order to cause lung disease, lymphadenopathy, and skin infection2. NTM pulmonary disease usually requires airway inflammation, ciliary dysfuction, abnormal sputum composition, and mucus plugging in order to trigger the distortion of bronchus and the decrease of ventilation2. It is classified into the cavitary form and bronchiectatic one according to its radiologic characteristics. The cavitary form of NTM pulmonary disease is more prevalent among older men with underlying chronic pulmonary disease. It is also accompanied by chronic obstructive pulmonary disease, cystic fibrosis, and bronchiectasis3,6. The bronchiectatic form is more commonly seen among elderly women with no predisposing factors4. Pleuritis is rare in cases of M. intracellulare infection10.
In our case, pleural effusion analysis showed an increased serum level of adenosine deaminase (ADA) and lymphocyte dominant exudate. After a presumptive diagnosis of tuberculous pleurisy, we started the standard treatment for it. Afterwards, M. intracellulare was identified using a polymerase chain reaction-restriction fragment length polymorphism method and liquid cultures of the pleural effusion and pleural biopsy. To our knowledge, M. intracellulare pleuritis proven by pleural effusion and pleural biopsy are rare. We experienced a case of M. intracellulare pleuritis proven by pleural effusion and pleural biopsy. Here, we report our case with a review of literature.
A 69-year-old man was admitted to our hospital with chief complaints of a 1-month history of dyspnea of Medical Research Council grade fourth, left pleuritic chest pain, anorexia, and general weakness. He experienced a 1-month history of aggravated cough and sputum. He had a 40 pack-year smoking history and a medical history of hypertension and idiopathic pulmonary fibrosis since the year of 2005. He had been treated with mucolytic drug (acetylcystein 600 mg t.i.d.) for one year. However, he had not been treated with immunosuppressive agents including steroid. In 2006, the patient underwent percutaneous coronary intervention for the right coronary artery. He denied any other past medical history.
On admission, He had vital signs such as blood pressure 104/66 mm Hg, pulse rate 80 beats/min, respiratory rate 26 breaths/min, and body temperature 36.9℃. On physical examination, He had an acute ill-looking appearance on the face. Chest auscultation revealed diminished breathing sounds on the left lower lung fields with fine crackles on the both lower lung fields. Other results of the physical examination were non-specific.
Laboratory findings showed white blood cell counts 8,200/mm3, hemoglobin 11.0 g/dL, platelet counts 396,000/mm3, aspartate aminotransferase 82 IU/L, alanine aminotransferase 64 IU/L, total bilirubin 0.7 mg/dL, and alkaline phosphatase 216 IU/L. Arterial blood gas analysis on room air yielded pH of 7.49, PaO2 of 65 mm Hg, PaCO2 of 33 mm Hg, and HCO3- of 25 mEq/L.
Chest radiography revealed the left pleural effusion and reticular densities in the bibasilar area (Figure 1). High-resolution computed tomography revealed a moderate amount of pleural effusion on the left side and multifocal ill-defined patchy reticular opacities with honeycombing lesions in both lower lobes of the lung (Figure 2).
To diagnose and treat this pleural effusion, he underwent a thoracentesis and pleural biopsy. On pleural effusion analysis, there were such findings as pale yellow color, specific gravity 1.010, red blood cell counts 1,100/mm3, white blood cell counts 1,800/mm3 with 4% neutrophils and 88% lymphocyte, pH 7.2, protein 5.4 g/dL, lactate dehydrogenase 282 U/L, glucose 73 mg/dL, albumin 2.5 g/dL, ADA 142 IU/L, negative acid-fast staining, non-specific cytology, and negative real-time polymerase chain reaction for M. tuberculosis. Pleural biopsy showed chronic granulomatous inflammation, but Ziehl-Neelsen stain failed to demonstrate the organism. Hence, he was tentatively diagnosed with tuberculous pleurisy. We prescribed isoniazid (300 mg/day), rifampicin (600 mg/day), ethambutol (1,200 mg/day) and pyrazinamide (1,500 mg/day). Following the initiation of standard treatment for tuberculosis, M. intracellulare was identified using a polymerase chain reaction-restriction fragment length polymorphism method11 and liquid cultures of the pleural effusion and pleural biopsy. Also, three consecutive sputum examination on admission revealed all negative Ziehl-Neelsen stain, but M. intracellulare was isolated in all liquid cultures one month later. Based on these findings, he was treated with azithromycin (250 mg/day), rifampicin (600 mg/day), and ethambutol (800 mg/day). One month later, chest radiograph showed that pleural effusion was improved on the left side (Figure 1). At a 1-month follow-up, the patient was clinically well. The patient is scheduled to receive the treatment for the next one and a half year.
NTM are commonly occurring organisms and have been recovered in many parts of the world and from a variety of environmental reservoirs including fresh and salt water, soil and biofilms3. The mode of transmission of NTM to humans has not been defined, although person-to-person transmission is thought not to occur or to be very uncommon, at least in immune-competent hosts3. Not all NTM are pathogenic for humans. NTM are usually less virulent than M. tuberculosis. But NTM are potential pathogens accompanied by diverse diseases. Pulmonary disease is the most common among them3. It is known that patients with pre-existing structural lung disease are at increased risk of non-tuberculous mycobacterial lung diseases. Respiratory diseases associated with risk of developing non-tuberculous mycobacterial lung diseases include chronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, previous tuberculosis, silicosis, pneumoconiosis, and alveolar proteinosis. Other established risk factors also include old age, male sex, smoking, alcohol abuse, residence in urban or coastal environments and participation in mining and smelting2. The signs and symptoms of NTM lung disease are generally non-specific and reflect the form of disease and comorbidities rather than the species of NTM involved. Chronic cough, sputum, and fatigue are very common. Fever and sweats are less frequent (<50% of patients). Malaise, hemoptysis, weight loss and wasting are uncommon and usually reflect advanced disease3.
Non-tuberculous mycobacterial lung disease is classified into cavitary form and bronchiectatic one according to its clinical and radiologic characteristics4,7. According to Koh et al.7, a total of 195 patients comprised 82 cases (62 men and 20 women, 42%) of upper lobe cavitary form, 101 cases (20 men and 81 women, 52%) of nodular bronchiectatic form and 12 cases (six men and six women, 6%) of unclassifiable variants. Cavitary form is more prevalent among older men with underlying chronic pulmonary disease. Common findings in the chest radiograph include upper lobe cavitary lesions and endobronchial spread evidenced by nodules adjacent to foci of disease, cicatricial atelectasis and pleural thickening4. Bronchiectatic form is more commonly seen among elderly women with no predisposing factor including smoking. Radiographic findings include bronchiectasis and small nodules in the right middle lobe and left lingular division. The clinical and radiologic features of NTM infection resemble those of tuberculosis. Pleural effusion is rare, however, in cases of NTM infection9,10. Currently, clarithromycin and azithromycin have become the cornerstones of therapy for M. intracellulare. In addition, rifampin, ethambutol, aminoglycoside and quinolone are also used for the treatment of M. intracellulare3.
Pleuritis caused by NTM is very rare10. Christensen et al.12 reviewed 100 patients with M. intracellulare lung disease and found that pleural effusions were unusual, occurring in 6 percent of patients with M. intracellulare. Shu et al.13 reported NTM pleurisy characteristics as below: 11.5% of the overall episodes of mycobacterial pleurisy are due to NTM recently; NTM pleurisy were less likely to have lung involvement; NTM pleurisy had a higher pleural effusion leukocyte count and a lower percentage of lymphocytes; NTM pleurisy were younger, and tended to have more extra-pleural involvement and immune dysfunction; one-year mortality was 37% and anti-NTM treatment was associated with better survival.
The exact pathogenesis of pleuritis due to NTM remains obscure. It is possible that NTM may gain entry to a pleural cavity containing an effusion of unrelated etiology through a transient bacteremia or by contiguous spread from a small subpleural focus and may grow in the pleural fluid without eliciting any readily discernible pathologic reaction14.
In conclusion, NTM accompanied by pleural effusion is rare. Physicians should consider, however, that M. intracellulare might cause pleuritis. It is therefore recommended that pleural effusion and pleural biopsy should be evaluated for M. intracellulare pleurisy when patients are suspected to have tuberculous pleurisy.
Figures and Tables
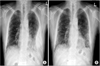 | Figure 1Chest X-ray findings. (A) On admission, chest X-ray revealed left pleural effusion with reticular densities in the bibasilar area. (B) At a 1-month follow-up, chest X-ray revealed improvement of left pleural effusion. |
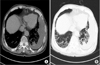 | Figure 2High-resolution computed tomography findings. (A) Mediastinal window setting revealed a moderate amount of left pleural effusion with subtle pleural thickening. (B) Lung window setting revealed multifocal, ill-defined, patchy reticular opacities with honeycombing lesions in both lower lobes of the lung. |
References
1. Murray MP, Laurenson IF, Hill AT. Outcomes of a standardized triple-drug regimen for the treatment of nontuberculous mycobacterial pulmonary infection. Clin Infect Dis. 2008. 47:222–224.
2. Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008. 31:1322–1333.
3. Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest. 2008. 133:243–251.
4. Martinez S, McAdams HP, Batchu CS. The many faces of pulmonary nontuberculous mycobacterial infection. AJR Am J Roentgenol. 2007. 189:177–186.
5. Fowler SJ, French J, Screaton NJ, Foweraker J, Condliffe A, Haworth CS, et al. Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J. 2006. 28:1204–1210.
6. Field SK, Cowie RL. Lung disease due to the more common nontuberculous mycobacteria. Chest. 2006. 129:1653–1672.
7. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–348.
8. Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, et al. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. J Korean Med Sci. 2005. 20:777–783.
9. Kwak SY, Bae SY, Yun WK, Kim MY, Kim YJ, Choi MK, et al. Mycobacterium intracellulare pulmonary infection accompanied with pleural effusion. Korean J Med. 2008. 75:475–478.
10. Kakugawa T, Mukae H, Kajiki S, Tanaka A, Yamayoshi T, Inoue M, et al. Mycobacterium avium pleuritis in a non-immunocompromised patient. Intern Med. 2008. 47:1727–1731.
11. Devallois A, Goh KS, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997. 35:2969–2973.
12. Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Anderson J, et al. Initial roentgenographic manifestations of pulmonary Mycobacterium tuberculosis, M kansasii, and M intracellularis infections. Chest. 1981. 80:132–136.
13. Shu CC, Lee LN, Wang JT, Chien YJ, Wang JY, Yu CJ, et al. Non-tuberculous mycobacterial pleurisy: an 8-year single-centre experience in Taiwan. Int J Tuberc Lung Dis. 2010. 14:635–641.
14. Gribetz AR, Damsker B, Marchevsky A, Bottone EJ. Nontuberculous mycobacteria in pleural fluid: assessment of clinical significance. Chest. 1985. 87:495–498.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download