Abstract
Background
Hemoptysis due to pulmonary tuberculosis (TB) frequently develops in Korea where the prevalence of TB is intermediate. The effect of bronchial artery embolization (BAE) on the control of massive hemoptysis has been well known. This study is designed to identify the risk factors contributing to rebleeding after BAE in patients with TB.
Methods
We retrospectively evaluated risk factors and the time for rebleeding after BAE in 72 patients presenting with hemoptysis.
Results
The overall immediate success rate of BAE was 93.1% (67 of 72 patients). Of the 29 patients (40.3%) who showed rebleeding after BAE, 13 patients experienced rebleeding within 1 month, and 14 patients between 1 month to 1 year. The existence of a shunt in angiographic finding, aspergilloma, and diabetes mellitus were risk factors of rebleeding after BAE in multivariate analysis.
Conclusion
BAE was very effective for obtaining immediate bleeding control in hemoptysis associated with active TB or post-TB sequelae. It is important to observe whether or not rebleeding occurs up to 1 year of BAE especially in TB patients with aspergilloma, DM, or a shunt. Even rebleeding can be managed well by second BAE.
Bronchial artery embolization (BAE) has been established as an effective and useful means to achieve treatment of chronic and recurrent hemoptysis, as well as immediate control of massive hemoptysis and to manage inoperable patients who had poor pulmonary function or chronic pulmonary diseases1-3. Although there are several causes of massive hemoptysis as known in other reports, tuberculosis (TB) is common cause especially in Korea4, where the prevalence of TB is intermediate, 149 rate per 100,000 population/yr compared with 15 rate per 100,000 population/yr in US in 20115.
Though BAE in TB patients has been studied previously6-9, the result of risk factors associated with TB activity were not consistent. So, we evaluated the characteristics of hemoptysis and tried to find the risk factors for rebleeding in patients who underwent BAE to control hemoptysis associated with active TB or post-TB sequelae.
We retrospectively reviewed consecutive 92 patients who underwent BAE due to hemoptysis in Respiratory Division, Department of Internal Medicine, Soonchunhyang University, Cheonan Hospital, from 1999 to 2008. This study was conducted in accordance with requirement of an Institutional Review Board for such retrospective analysis of medical records.
The clinical records of all patients were assessed retrospectively, and the following data and images were collected to analyze the activity of TB, recurrence rate, and risk factor for rebleeding: age, sex, clinical features, past history, laboratory findings, sputum study, embolization material, angiographic images, chest roentgenography, chest CT scan, and bronchoscopy.
All patients were categorized as active TB or post-TB. Acitve TB was defined on the basis of acid-fast bacilli (AFB) positive, clinical suspicion or imaging including consolidation, endobronchial spread pattern, or tree-in-bud opacities. TB sequelae was defined on the basis of previous history of TB and AFB-negative with imaging including bronchiectasis, calcified nodules or fibrosis10. TB destroyed lung was defined as parenchymal damage to more than one lung lobe due to previous pulmonary TB, but no recent evidence of active TB11. Multi-drug resistant TB (MDR TB) was defined as resistance to both isoniazid and rifampicin, with or without resistance to any other antituberculous drugs. Immediate control of bleeding was defined as a cessation of bleeding obtained without recurrence within 24 hours of successful BAE12. Rebleedings, which was defined as recurrence and/or persistence of bleeding after immediate control, are categorized into two groups according to the time: early-onset, within 1 month; and late-onset, beyond 1 month. Patients were classified according to the amount of greater than 200 mL on admission13. Shunt means pulmonary-bronchial artery shunt in angiographic findings.
Our conservative medical measures included strict bed rest, nothing by mouth, hemostatics, monitoring of oxygen saturation, respiratory rate, heart rate and blood pressure, the supply of oxygen if needed. Anti-tuberculous medication included isoniazid, rifampin, ethambutol, and pyrazinamide.
A standardized BAE procedure was used as follows: a catheter was introduced into the right femoral artery through an introducer sheath using the Seldinger technique3. Selective bronchial artery angiography was then performed. Embolization was performed when the bronchial arteries appeared to be the source of hemoptysis (tortuous hypertrophy, systemic-to-pulmonary shunt, extravasation of contrast material, or peribronchial hypervascularisation)14. Agents used for embolization included coils, gelform, polyvinyl alcohol or combination during study period.
Data were analysed using the Statistical Package for the Social Sciences version 14.0 (SPSS Inc., Chicago, IL, USA). The groups were compared using Student's t-test or the Mann-Whitney U test for continuous variables and χ2 test or Fisher's exact test for categorical variables to find the risk factor for recurrence after BAE. Multivariate analysis was obtained using logistic regression. The Kaplan-Meier survival method was used to estimate their recurrence-free probability of the patients after BAE. Cox's regressional hazards model was used to find the independent factors of recurrence-free time. A p<0.05 was considered statistically significant.
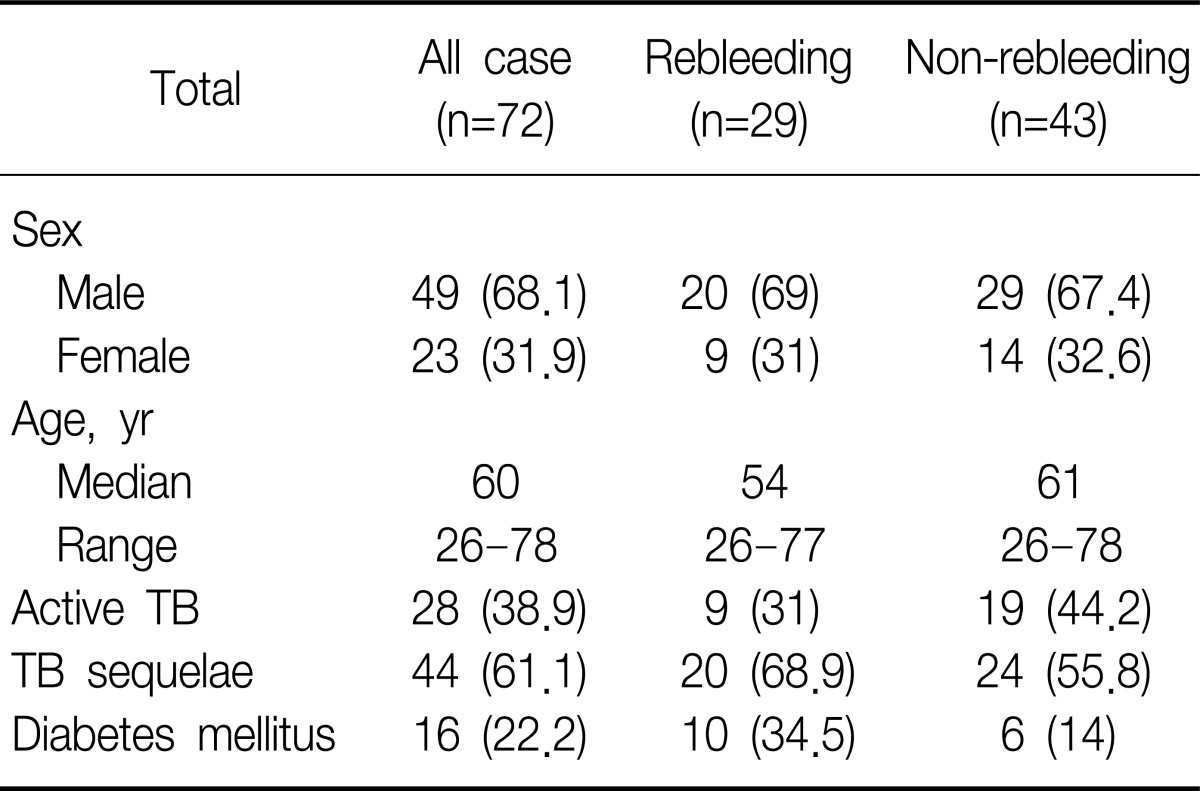
We retrospectively reviewed consecutive 92 patients who underwent BAE due to hemoptysis. Twenty cases were excluded in which hemoptysis was not associated with activity or sequelae of TB: metastatic tumor lesion (n=1), emphysema (n=1), leukemia (n=1), lung mass (n=4), pneumonia (n=1), and equivocal cases (n=12). The patients consisted of 72 (49 men, 68.1%; median age, 60 years; range, 26 to 78 years) (Table 1).
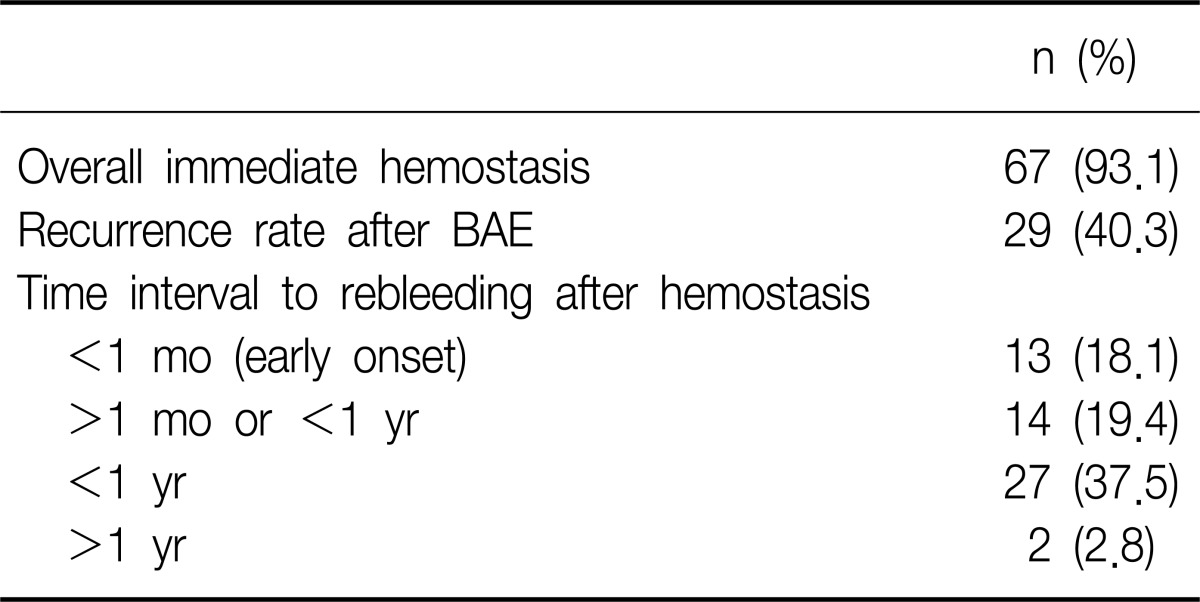
The mean amount of bleeding at initial presentation was 210 mL (range, 20 to 2,000 mL). Overall immediate success rate of BAE was 93.1% (67 of 72). The rebleeding group consisted of 29 (40.3%) and the non-rebleeding group 43 patients (59.7%). The patients with successful BAE have undergone follow-up for median 13 months (range, 1 to 63 months). Among 29 patients of the rebleeding group, 13 patients experienced recurrence within 1 month, and 14 patients between 1 month to 1 year. Only two cases showed rebleeding beyond 1 year after initial hemostasis by BAE: bronchiectasis (n=1) and TB destroyed lung (n=1) managed by conservative medication and BAE, respectively. So, rebleeding after BAE was shown in 27 of 72 cases within 1 year (Table 2).
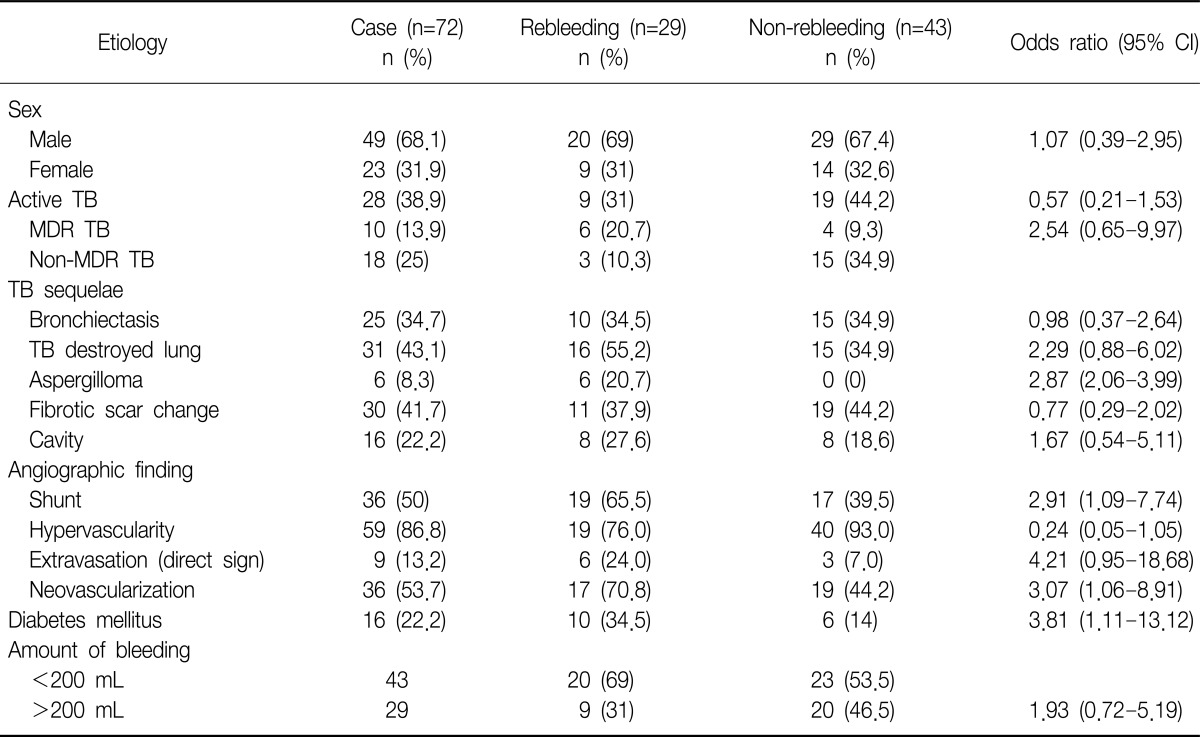
Active TB patients were 38.9% (n=28) including MDR TB (n=10). Post-TB sequelae included bronchiectasis, TB destroyed lung, aspergilloma, or fibrotic scar change (Table 3). Among five patients who failed to stop bleeding by BAE immediately, two patients died: one patient with active MDR TB showed massive rebleeding died despite second BAE within 30 days after first BAE; another patient with active non-MDR TB had massive rebleeding and expired despite second BAE within 60 days after BAE. In subgroup of 28 patients with active TB, MDR TB showed significantly higher rebleeding than non MDR TB (odds ratio [OR], 7.5; 95% confidence interval [CI], 1.276 to 44.085; p<0.035). Active TB group excluding MDR TB was significantly associated with non-rebleeding (OR, 0.240; 95% CI, 0.061 to 0.949; p=0.044).
The rate of recurrence based on the underlying pulmonary disease was 40% (10 of 25) for bronchiectasis, 51.6% (16 of 31) for destroyed lung, 100% (6 of 6) for aspergilloma, 36.7% (11 of 30) for fibrotic scar change, and 32.1% (9 of 28) for active TB. Active TB was not associated with risk of rebleeding (OR, 0.57; 95% CI, 0.21 to 1.53; p>0.05). Bronchiectasis, TB destroyed lung, fibrotic change, cavity, age, sex, or amount of hemoptysis were not associated with the risk of recurrence of bleeding (Table 3).
Recurrence rates in the suspected bleeding site based on direct and/or indirect sign in angiographic finding were 50% (15 of 30) for right lung, 21% (4 of 19) for left lung, and 39% (7 of 18) for both lung (p=0.315). Shunt and neovascularization of angiographic finding were significantly different between rebleeding and non-rebleeding group (OR, 2.91; 95% CI, 1.09 to 7.74; p<0.031 and OR, 3.07; 95% CI, 1.06 to 8.91; p<0.036, respectively). The findings of nonbronchial systemic artery in angiographic examination were not risk factors for rebleeding. Findings of extravasations as direct sign in angiography (the pathognomonic sign of hemorrhage)3 were not associated with rebleeding after BAE (OR, 4.21; 95% CI, 0.95 to 18.68).
There were no significant factors which can influence on difference between early-onset group and late-onset group among active TB, destroyed lung, fibrotic change, aspergilloma, diabetes mellitus, shunt, age, sex, amount of bleeding, and embolization material.
Diabetes mellitus (DM) was the factor significantly associated with a risk of rebleeding (OR, 3.81; 95% CI, 1.11 to 13.12). Of 16 patients with DM, 10 patients (62.5%) showed recurrent bleeding: 6 patients (60%) within 1 month and 4 (40%) after 1 month. Among these 10 patients, 4 patients (40%) had active TB, 2 (20%) destroyed lung, 2 (20%) fibrotic change, 1 (10%) bronchiectasis, and 1 (10%) aspergilloma.
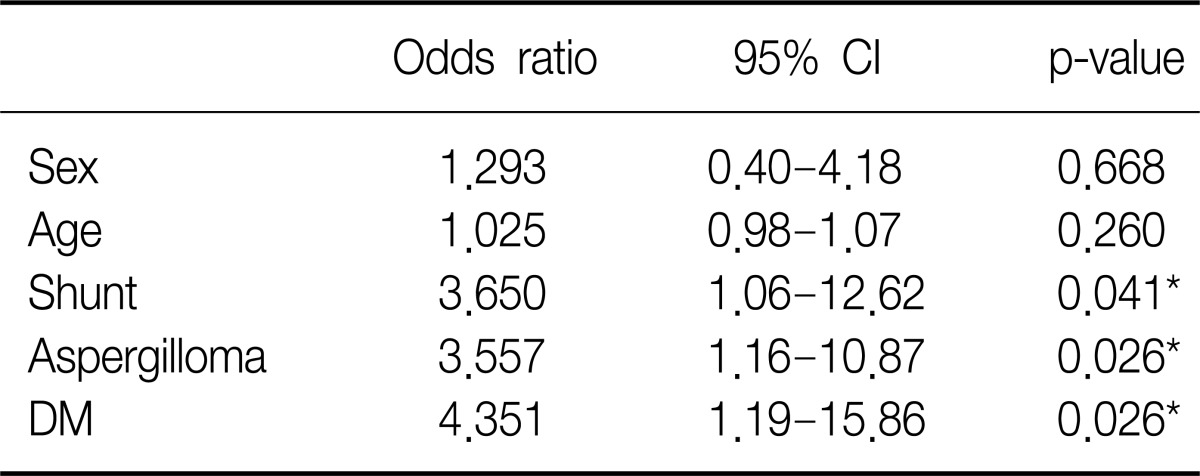
Aspergilloma were significantly associated with rebleeding after BAE (p=0.003). Aspergillom, DM and existence of shunt in angiographic finding were the risk factors of recurrence after BAE in multivariate logistic regression analysis (Table 4).
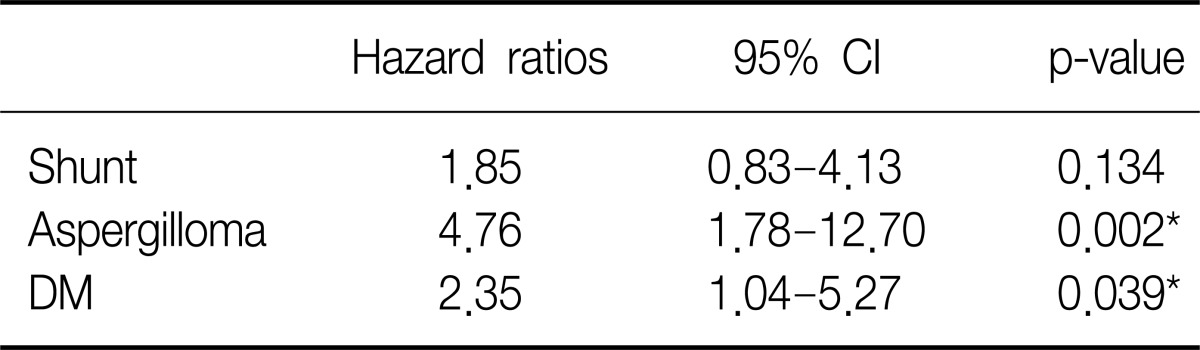
Cumulative hemoptysis non-recurrence rate after BAE were 84.7% for 1 month, 76.2% for 2 months, 71.4% for 6 months, 59.4% for 12 months, 55.7% for 24 months, and 50.6% for 36 months. Recurrence-free time was significantly correlated with shunt in angiographic finding (p=0.016) (Figure 1), DM (p=0.035) (Figure 2), and aspergilloma (p<0.01). TB activity was not associated with recurrence-free time (p=0.315) (Figure 3). Independent factors of recurrence-free time after BAE in multivariate analysis using Cox's regressional hazards model were aspergilloma and DM (Table 5).
The subsequent procedures for control of recurrent bleeding are as follows: medical treatment in 15 patients, repeated BAE in 13, operation in one. Complications arising after BAE included: chest pain (n=10), fever (n=4), headache (n=4), cough (n=2), hiccup (n=2), back pain (n=1), urticaria (n=1), and nausea (n=1). Three patients who underwent surgery to manage the rebleeding after BAE include: one with TB, one with fibrotic change, and one with destroyed lung.
Three patients who necessitated mechanical ventilation, showed late recurrences: TB (n=2), one of them resulting in death two months after initial BAE; bronchiectasis (n=1).
Seven patients, who expired during follow-up post procedure, include 4 patients in rebleeding group and 3 patients in non-bleeding group. Four patients who expired in rebleeding group are as follows: one patient with TB expired from pneumonia with septic shock in 48 months, two patients with TB from massive hemoptysis within 2 months of BAE; one patient with aspergilloma (n=1) from massive hemoptysis in 6 months. Three patients who expired in non-rebleeding group are as follows: destroyed lung (n=2), one from respiratory failure in 12 months, one from pneumonia with acute respiratory distress syndrome in 30 months; fibrosis (n=1), one from respiratory failure without recurrence (Figure 4).
Hemoptysis is one of the most common symptoms in patient with respiratory disease and may be a life-threatening condition. Massive hemoptysis occurs in approximately 1.5% of patients presenting with hemoptysis. The prognosis is poor, and the mortality by bleeding or asphyxia reaches 50-60%15,16.
BAE is now recognized as the first-line treatment for control of hemoptysis17,18. In our study, immediate success rate was 93.1% (67 of 72 patients), which was similar to 94% in a report by Swanson et al.19 compared with earlier report ranged from 75% to 90%16,20. In the past, the control of hemoptysis has been obtained using surgical methods including pneumonectomy or lobectomy21. The main advantage is that surgery can remove the source of bleeding. Massive hemoptysis made it difficult to localize the bleeding point, in which case emergency surgery is associated with a high mortality rate 17-35%16. Indications for BAE include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and elevated surgical risk. Recent surveys suggest a shift from surgery to BAE as a first-line procedure in severe hemoptysis17,18.
Recurrent bleeding despite apparently adequate embolotherapy remains a considerable problem with rebleeding occurring in 9% to 42% of patients2,7,19-22, which is similar to recurrence rate of 40.3% (29 of 72) in this report.
We intended to assess risk factors that affected the rebleeding in patient with TB or post-TB sequelae in country where TB prevalence is intermediate, because active TB and bronchiectasis were known as the most common etiologies of hemoptysis21,22. Lee et al.23 showed a significant association between active TB and recurrence after BAE. Active, persistent mucosal inflammation, which is a factor identified by Mossi et al.24 that might lead to recurrence of bleeding, would also be present in airways with active tuberculosis infection. On the other hand, van den Heuvel et al.8 reported that the presence of active TB amenable to treatment is protective. Chun and Belli25 showed the favourable outcomes for patients with active TB. Kato et al.26 reported similar findings with no recurrent cases in their active TB group. Our study showed no association between active TB and recurrence after BAE. In terms of medical treatment, patient with active TB was managed adequately in our study.
Besides being a feature of active TB, haemoptysis can also be a manifestation of complications such as cavitation, fibrosis, bronchiectasis, and mycetoma. In our study, bronchiectasis or destroyed lungs associated with TB were not associated with the risk of rebleeding. But shunt in angiographic finding was significantly associated with rebleeding. So, it could mean that shunt reflect significant decreased circulation to the destroyed site with inflammation and vascular exposure rather than bronchiectasis or destroyed lung per se27,28. In our study, event time doesn't coincide with second peak for rebleeding which reflects the recruitment of blood supply and neovascularization in 1 to 2 years after BAE which Hayakawa et al.2 described as the second peak. Although the initial amount of hemoptysis on admission or extravasation as direct sign in angiographic finding were expected to be risk factors for rebleeding, there was no significant association between the volume of hemoptysis and recurrence as in other reports7,23.
Shunt (36 of 72) or neovascularization (36 of 72) were not associated with death. Shunt was found in 4 of 7 patients who died, and neovasculization in 5 of 7 patients. In patients with shunt or neovascularization in angiographic finding, rebleeding can be managed well by 2nd BAE rather than definitive surgical management. Active TB was not associated with the risk for rebleeding, which means that active TB with hemoptysis can be treated with BAE8 accompanied by anti-tuberculous medication.
The most unfavourable results were shown in patients with aspergilloma, all six of whom suffered rebleeding (p<0.05). High recurrence rates for patients with aspergilloma have been shown in previous report7,22. It was shown that patients with aspergilloma often have extensive parasite circulation from different sources, and is likely to be associated with vasculitis in cases of thick vascular cavitary walls15,27. In our study, DM was shown to be significantly associated with recurrence after BAE, unlike previous reports to our knowledge. Some study showed increasing cavitation together with increased smear positivity in the diabetic group29,30. It is known that DM causes a decrement in lymphocyte activity and a diminution in the number of monocytes and macrophages31. One recent study has reported low plasma concentrations of rifampicin in diabetic patients with TB32. Even though there are conflicting reports regarding the influence of associated DM on the treatment outcome of TB patient, one study showed that type 2 DM was an independent and significant risk factor associated with an unfavorable outcome29. This suggests that clinicians must pay attention to possible recurrent hemoptysis in TB patient with associated DM.
It was known BAE was associated with a 5% rate of complications19. In our report, complications arising after BAE resolved spontaneously or with oral analgesics. Other major complications, such as spinal cord ischemia or mediastinal structure necrosis were not encountered. There was no significant correlation between rebleeding and the use of any particular agents.
In our study, of the 29 patients with rebleeding, 14 patients (48.3%) underwent a second BAE, 12 (41.4%) received supportive medical treatment and three (10.3%) with fibrotic change received surgery. So, most patient with rebleeding (26 of 29, 89.7%) were managed by second BAE or medical treatment with preserving surgery.
The overall mortality rate was 9.7% (7 of 72): 13.8% (4/29) in rebleeding group and 7% (3 of 43) in non-rebleeding group, compared with overall mortality of 13% reported in other study33.
The limitations of our study are related to its retrospective nature and to the fact that it was conducted in one referral center. Nevertheless, our study may provide very useful information for risk assessment and the proper management of rebleeding after BAE in patient with pulmonary disease associated with TB.
In conclusion, BAE was very useful in obtaining immediate bleeding control which was slightly better than that in other report. It is important to observe rebleeding within 1 year of BAE especially in patients with aspergilloma and/or DM which are important risk factors to predict rebleeding after BAE. Even rebleeding after BAE can be managed well by second BAE. Those patients should be evaluated who are definitive surgical candidate after undertaking successful BAE in further study.
References
1. Garzon AA, Gourin A. Surgical management of massive hemoptysis: a ten-year experience. Ann Surg. 1978; 187:267–271. PMID: 637582.
2. Hayakawa K, Tanaka F, Torizuka T, Mitsumori M, Okuno Y, Matsui A, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992; 15:154–158. PMID: 1628281.

3. Rabkin JE, Astafjev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987; 163:361–365. PMID: 3562815.

4. Kim BC, Kim JM, Kim YS, Kim SM, Choi WY, Lee KS, et al. Effect of bronchial artery embolization in the management of massive hemoptysis: factors influencing rebleeding. Tuberc Respir Dis. 1996; 43:590–599.
5. World Health Organization. TB country profile for Rep. Korea [Internet]. 2011. cited 2013 Jan 1. Geneva: World Health Organization;Available from: http://www.whoint/countries/kor/en.2011.
6. Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996; 200:691–694. PMID: 8756916.

7. Kim YG, Yoon HK, Ko GY, Lim CM, Kim WD, Koh Y. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Respirology. 2006; 11:776–781. PMID: 17052307.

8. van den Heuvel MM, Els Z, Koegelenberg CF, Naidu KM, Bolliger CT, Diacon AH. Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis. 2007; 11:909–914. PMID: 17705959.
9. Gross AM, Diacon AH, van den Heuvel MM, Janse van Rensburg J, Harris D, Bolliger CT. Management of life-threatening haemoptysis in an area of high tuberculosis incidence. Int J Tuberc Lung Dis. 2009; 13:875–880. PMID: 19555538.
10. Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, et al. Pulmonary tuberculosis: CT findings. Early active disease and sequential change with antituberculous therapy. Radiology. 1993; 186:653–660. PMID: 8430169.
11. Bobrowitz ID, Rodescu D, Marcus H, Abeles H. The destroyed tuberculous lung. Scand J Respir Dis. 1974; 55:82–88. PMID: 4854707.
12. Cremaschi P, Nascimbene C, Vitulo P, Catanese C, Rota L, Barazzoni GC, et al. Therapeutic embolization of bronchial artery: a successful treatment in 209 cases of relapse hemoptysis. Angiology. 1993; 44:295–299. PMID: 8457080.

13. Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987; 294:301–309. PMID: 3425580.

14. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002; 22:1395–1409. PMID: 12432111.

15. Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchial artery embolization. Radiology. 1983; 146:627–634. PMID: 6828674.

16. Rémy J, Voisin C, Dupuis C, Beguery P, Tonnel AB, Denies JL, et al. [Treatment of hemoptysis by embolization of the systemic circulation]. Ann Radiol (Paris). 1974; 17:5–16. PMID: 4820232.
17. Haponik EF, Fein A, Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000; 118:1431–1435. PMID: 11083697.
18. Fartoukh M, Khalil A, Louis L, Carette MF, Bazelly B, Cadranel J, et al. An integrated approach to diagnosis and management of severe haemoptysis in patients admitted to the intensive care unit: a case series from a referral centre. Respir Res. 2007; 8:11. PMID: 17302979.

19. Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization : experience with 54 patients. Chest. 2002; 121:789–795. PMID: 11888961.
20. Uflacker R, Kaemmerer A, Picon PD, Rizzon CF, Neves CM, Oliveira ES, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985; 157:637–644. PMID: 4059552.

21. Mal H, Rullon I, Mellot F, Brugiere O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999; 115:996–1001. PMID: 10208199.

22. Remy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977; 122:33–37. PMID: 830351.

23. Lee S, Chan JW, Chan SC, Chan YH, Kwan TL, Chan MK, et al. Bronchial artery embolisation can be equally safe and effective in the management of chronic recurrent haemoptysis. Hong Kong Med J. 2008; 14:14–20. PMID: 18239238.
24. Mossi F, Maroldi R, Battaglia G, Pinotti G, Tassi G. Indicators predictive of success of embolisation: analysis of 88 patients with haemoptysis. Radiol Med. 2003; 105:48–55. PMID: 12700545.
25. Chun JY, Belli AM. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010; 20:558–565. PMID: 19727742.

26. Kato A, Kudo S, Matsumoto K, Fukahori T, Shimizu T, Uchino A, et al. Bronchial artery embolization for hemoptysis due to benign diseases: immediate and longterm results. Cardiovasc Intervent Radiol. 2000; 23:351–357. PMID: 11060364.

27. Stoll JF, Bettmann MA. Bronchial artery embolization to control hemoptysis: a review. Cardiovasc Intervent Radiol. 1988; 11:263–269. PMID: 3145138.

28. Osaki S, Nakanishi Y, Wataya H, Takayama K, Inoue K, Takaki Y, et al. Prognosis of bronchial artery embolization in the management of hemoptysis. Respiration. 2000; 67:412–416. PMID: 10940796.

29. Wang CS, Yang CJ, Chen HC, Chuang SH, Chong IW, Hwang JJ, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009; 137:203–210. PMID: 18559125.

30. Hendy M, Stableforth D. The effect of established diabetes mellitus on the presentation of infiltrative pulmonary tuberculosis in the immigrant Asian community of an inner city area of the United Kingdom. Br J Dis Chest. 1983; 77:87–90. PMID: 6860557.

31. Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus: pneumonia. Infect Dis Clin North Am. 1995; 9:65–96. PMID: 7769221.
32. Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006; 43:848–854. PMID: 16941365.

33. Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med. 2003; 29:317–320. PMID: 12594593.

Figure 1
The cumulative hemoptysis control rate as depicted graphically by the Kaplan-Meier method: p=0.016; shunt (+) vs. shunt (-). BAE: bronchial artery embolization.

Figure 2
The cumulative hemoptysis control rate as depicted graphically by the Kaplan-Meier method: p=0.035; DM (+) vs. DM (-). DM: diabetes mellitus; BAE: bronchial artery embolization.
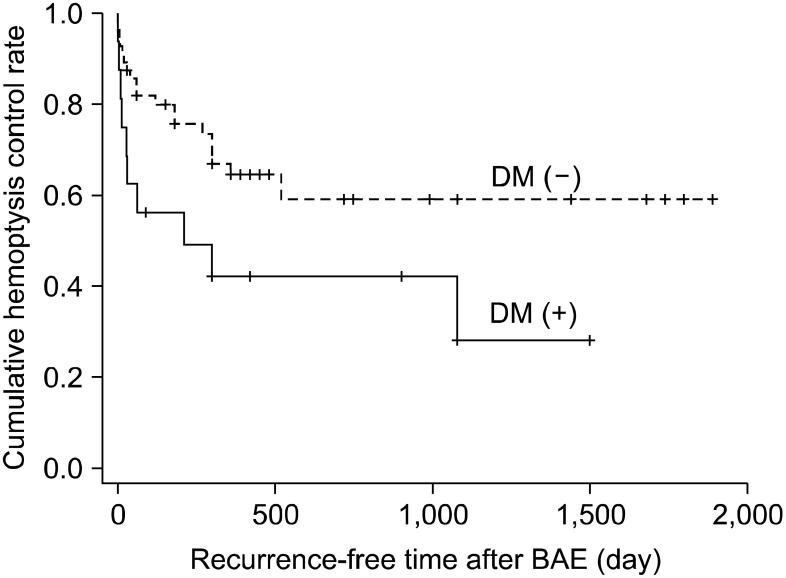
Figure 3
The cumulative hemoptysis control rate as depicted graphically by the Kaplan-Meier method: p=0.315; TB vs. post TB sequelae. TB: tuberculosis; BAE: bronchial artery embolization.

Figure 4
Flowchart of management and outcome after bronchial artery embolization in patients with hemoptysis. *Three patients died of massive hemoptysis.
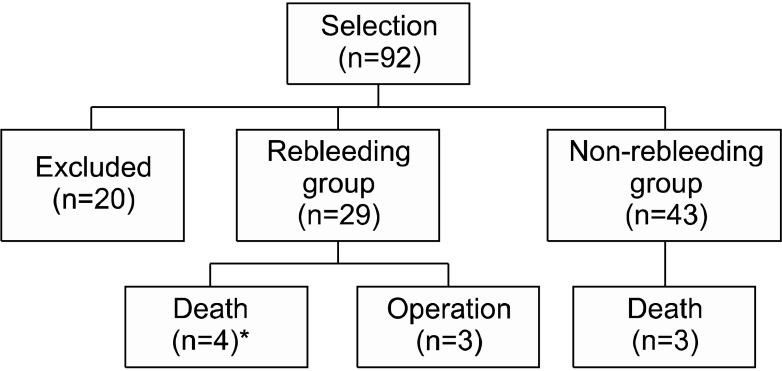
Table 3
Analysis of risk factors affecting rebleeding after immediate bleeding control with bronchial artery embolization




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download