Abstract
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type (extranodal MZL) is a distinct subgroup of non-Hodgkin's lymphoma. Pulmonary extranodal MZL is a rare entity and accounts for less than 0.5% of primary pulmonary malignancies. Only a few cases of simultaneous occurrence of lung cancer and pulmonary extranodal MZL have been reported. A 60-year-old woman was referred to our hospital with a pulmonary nodule. She was diagnosed with lung adenocarcinoma by percutaneous needle biopsy. The protrusions into the left main bronchus were found by accident while performing bronchoscopy during lung cancer evaluation. The bronchial lesions were diagnosed as extranodal MZL. Although the patient underwent surgical resection for the lung adenocarcinoma, the pulmonary extranodal MZL was left untreated; it was monitored during follow-up visits. To our knowledge, this is the first report of synchronous lung adenocarcinoma and primary extranodal MZL of the main bronchus.
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type (extranodal MZL), also called MALT lymphoma, is a distinct subgroup of non-Hodgkin's lymphoma (NHL) that constitutes about 5% of all NHLs1,2. Although the stomach is involved most frequently, the lung is one of the most common sites among non-gastrointestinal sites in extranodal MZL3,4. More than two thirds of primary pulmonary NHLs are extranodal MZLs but they account for less than 0.5% of all primary lung neoplasms5-7.
The simultaneous occurrence of gastric adenocarcinoma and gastric extranodal MZL has often been documented8. However, only a few cases of simultaneous lung cancer and pulmonary extranodal MZL have been reported9-11. Also, it is extremely rare that primary extranodal MZL occurs in the large airways12. To our knowledge, only one case of lung cancer coexisting with extranodal MZL of the large airway has been reported9. We report a case of synchronous lung adenocarcinoma and primary extranodal MZL of the left main bronchus.
A 60-year-old woman was referred to our hospital with a pulmonary nodule in the right upper lung seen on chest radiograph during a routine health checkup. She had been treated curatively with surgical resection for papillary thyroid carcinoma 3 years ago. She had never smoked. There was no history of respiratory illness such as tuberculosis. Her vital signs were normal, and physical examination revealed no abnormalities. Laboratory data were within the normal range except a tumor marker, carcinoembryonic antigen, which had a level of 6.07 ng/mL. Pulmonary function testing revealed no ventilatory defect.
A chest radiograph showed a nodule in the right upper lung (Figure 1A). A chest computed tomographic (CT) scan showed a peripherally located 2.4×1.8 cm sized solid nodule in the anterior segment of the right upper lobe (Figure 1B). Fluorine 18-labeled fluorodeoxyglucose (FDG)-positron emission tomography-CT scan revealed abnormal FDG uptake in the right upper lobe lung nodule and in the right hilar lymph node (Figure 2). She underwent CT image-guided percutaneous core needle biopsy of the nodule in the right upper lobe. Additionally, a flexible bronchoscopic examination was performed to identify the endobronchial lesion. At bronchoscopy, a few small variable-sized protrusions were observed in the proximal left main bronchus and a biopsy was performed (Figure 3).
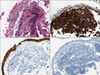
The needle biopsy specimens of the right lung nodule showed non-mucinous adenocarcinoma with a lepidic and acinar growth pattern (Figure 4A). Immunohistochemical stains were positive for cytokeratin (CK) 7 and thyroid transcription factor-1, and negative for CK20 (Figure 4B~D). Histopathology of the bronchoscopic biopsy specimens of the left main bronchus lesions showed diffuse proliferation of atypical lymphoid cells beneath the mucosal epithelium (Figure 5A). Immunohistochemical stains showed diffuse strong positive for CD20, and were negative for pan cytokeratin and CD10 (Figure 5B~D). The proliferation fraction (Ki67) was very low (<5%). The bronchial lesion was diagnosed as extranodal MZL. A bone scan and a brain magnetic resonance imaging study found no other abnormal lesion. A gastroduodenoscopy revealed no mucosal abnormality.
She underwent right upper lobectomy and lymph node dissection for the lung adenocarcinoma. However, she did not receive specialized therapy for extranodal MZL of the left main bronchus. Since then she has been observed regularly.
In 1983, Isaacson and Wright13 first described two cases of MALT lymphoma arising in the gastrointestinal tract. Recently, it has been reclassified as extranodal marginal zone B-cell lymphoma of MALT type in the revised European-American classification of lymphoid neoplasms and the World Health Organization classification2,4,7.
Extranodal MZL is an extranodal lymphoma with 'low grade' features that infiltrates the marginal zone of reactive B-cell follicles. Extranodal MZL arises from the MALT of the stomach most frequently and also from lung, salivary gland, and thyroid gland2,4,7.
Pulmonary extranodal MZL is a rare entity and accounts for less than 0.5% of primary pulmonary malignancies, and less than 1% of all lymphomas5-7. Lung adenocarcinoma usually arises from peripheral small bronchi or alveolar epithelial cells and is increasing in incidence globally.
Synchronous primary malignancy and extranodal MZL in the same anatomical sites, such as stomach and lung is very unusual8-11. To our knowledge, there is only one other report showing the coexistence of lung cancer and pulmonary extranodal MZL of the large airway9. However, in that case, the histology of lung cancer was squamous cell carcinoma9. The present case is the first report of the simultaneous occurrence of lung adenocarcinoma and pulmonary extranodal MZL of the large airway.
The pathogenesis of extranodal MZL may be associated with chronic immune stimulation driven by infection or autoimmune disorder. The strong association between Helicobacter pylori and gastric extranodal MZL is known2,4. However, the etiology of pulmonary extranodal MZL is unclear7.
The simultaneous occurrence of adenocarcinoma and extranodal MZL in the stomach is well established. H. pylori seems to be one of the common causes of both diseases8.
The cause of the synchronous lesions in our case is uncertain. We postulate that a pulmonary extranodal MZL may coincide with a lung adenocarcinoma by accident. Little is known about the mechanism of the simultaneous occurrence of these different tumors. Moreover, the two different malignancies were located in different lungs in this case. However, other possible causes may include common etiologic factors and genetic or epigenetic alterations participating in the development of both the lung adenocarcinoma and extranodal MZL. One case report speculated that smoking could lead to both lung cancer and extranodal MZL9. Another report suggested that the API2-MALT1 fusion gene or factors associated with the gene may play a role in the development of both diseases11. In the current case, the patient had never smoked, and no examination for genetic change was performed. Further studies will be required to clarify the relationship between lung adenocarcinoma and pulmonary extranodal MZL. It is necessary to accumulate more cases with both malignancies.
Approximately half of the patients are asymptomatic at presentation, with abnormal radiologic findings being discovered on chest radiograph. Symptomatic patients present with nonspecific pulmonary symptoms, including cough, dyspnea, chest pain and hemoptysis. B symptoms are uncommon and observed in only a few patients4,7,14,15. In this current case, the patient with lung cancer was asymptomatic and the protrusions into the left main bronchus were found by accident while performing bronchoscopy during lung cancer evaluation.
The major radiographic patterns of pulmonary extranodal MZL have been reported as nodules, consolidation, ground-glass opacity, and centrilobular nodules with linear branching opacities (tree-in-bud sign)4,7,15,16. In the one published report on CT findings, single or multiple nodules or areas of consolidation were the main patterns, while none exhibited involvement of the main bronchus16. In the most recent Korean study, of the 61 enrolled patients, only two had masses in the main bronchus14. Therefore, our case is unusual because the patient presented with endobronchial protrusions without lung parenchymal lesions on bronchoscopy.
Although diagnosis can be achieved by bronchoscopic or transbronchial biopsy or by percutaneous needle biopsy, surgical lung biopsy is required in many cases 4,7,14,15.
Extranodal MZL is characterized by an infiltrate of small to medium-sized lymphocytes with irregular nuclei and abundant cytoplasm. Reactive follicles are usually observed. Lymphoepithelial lesions, in which tumor cells infiltrate the bronchial, bronchiolar, and alveolar epithelium, are characteristic but not pathognomonic2,4,7.
The tumor cells are positive for B cell markers (CD19, CD20, CD22, CD79a) and complement receptors (CD21 and CD35), and are usually negative for CD5, CD10, and CD23. The proliferation fraction (Ki67) is usually very low (<10%). Immunophenotyping studies are useful in confirming diagnosis, particularly when done on small biopsy specimens, and in differentiating this malignancy from diffuse large B cell lymphoma, small lymphocytic lymphoma, mantle cell lymphoma and follicular lymphoma2,4,7,13.
There is controversy about the optimal treatment of extranodal MZL; treatment ranges from observation only to surgery, radiotherapy or chemotherapy alone or in combination. Surgical resection and radiotherapy are preferred for localized cases and chemotherapy may be considered for bulky or disseminated cases. However, treatment protocols have not yet been established. If the patient is asymptomatic, the treatment may be a watch-and-wait policy instead of attempting immediate curative treatment4,7,14,15.
Pulmonary extranodal MZL is an indolent disease and has a favorable prognosis. The five-year survival rate is about 90%4,7,14. Extrapulmonary lesions and lymph node involvement are poor prognostic factors14.
In the present case, although the patient underwent surgical resection for the lung adenocarcinoma, the pulmonary extranodal MZL was left untreated, and monitored during follow-up visits.
Here, we report a unique case of pulmonary extranodal MZL of main bronchus coexisting with lung adenocarcinoma. The patient was asymptomatic and was diagnosed with pulmonary extranodal MZL by chance during investigation of an endobronchial lesion.
Figures and Tables
Figure 1
(A) Chest radiograph shows a nodule in the right upper lung. (B) Chest computed tomographic scan shows a 2.4×1.8 cm sized nodule in the anterior segment of the right upper lobe.
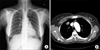
Figure 2
Fluorine 18-labeled fluorodeoxyglucose-positron emission tomography-computed tomographic scan shows a hypermetabolic nodule in the right upper lobe (SUVmax, 11.2).
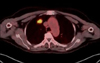
Figure 3
Bronchoscopic findings show a few small variable-sized protrusions in the proximal left main bronchus.
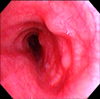
Figure 4
The computed tomography-guided percutaneous needle biopsy specimens of the right lung nodule. (A) Lung tumor tissue shows non-mucinous adenocarcinoma with a lepdic and acinar growth pattern and stromal infiltration. Immunohistochemical stains were positive for (B) cytokeratin (CK) 7 and (C) thyroid transcription factor-1, and negative for (D) CK20 (A~D, ×200).
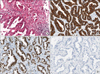
Figure 5
The bronchoscopic biopsy specimens of the left main bronchial lesions. (A) The bronchial mucosa shows diffuse proliferation of atypical lymphoid cells beneath the mucosal epithelium. The neoplastic lymphoid cells show diffuse strong positive for (B) CD20, but negative for (C) pan cytokeratin and (D) CD10 (A~D, ×200).
References
1. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998. 16:2780–2795.
2. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.
3. Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003. 101:2489–2495.
4. Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. 2001. Lyon: IARC Press.
5. Ahmed S, Siddiqui AK, Rai KR. Low-grade B-cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest. 2002. 20:1059–1068.
6. Koss MN. Malignant and benign lymphoid lesions of the lung. Ann Diagn Pathol. 2004. 8:167–187.
7. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. 2004. Lyon: IARC Press.
8. Wotherspoon AC, Isaacson PG. Synchronous adenocarcinoma and low grade B-cell lymphoma of mucosa associated lymphoid tissue (MALT) of the stomach. Histopathology. 1995. 27:325–331.
9. Suzuki T, Akizawa T, Suzuki H, Kitazume K, Omine M, Mitsuya T. Primary tracheal mucosa-associated lymphoid tissue lymphoma accompanying lung cancer. Common tumorigenesis or coincidental coexistence? Jpn J Thorac Cardiovasc Surg. 2000. 48:817–819.
10. Chanel S, Burke L, Fiche M, Molina T, Lerochais JP, Icard P, et al. Synchronous pulmonary adenocarcinoma and extranodal marginal zone/low-grade B-cell lymphoma of MALT type. Hum Pathol. 2001. 32:129–132.
11. Ichihara E, Tabata M, Takigawa N, Sato Y, Kondo E, Aoe M, et al. Synchronous pulmonary MALT lymphoma and pulmonary adenocarcinoma after metachronous gastric MALT lymphoma and gastric adenocarcinoma. J Thorac Oncol. 2008. 3:1362–1363.
12. Solomonov A, Zuckerman T, Goralnik L, Ben-Arieh Y, Rowe JM, Yigla M. Non-Hodgkin's lymphoma presenting as an endobronchial tumor: report of eight cases and literature review. Am J Hematol. 2008. 83:416–419.
13. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983. 52:1410–1416.
14. Oh SY, Kim WS, Kim JS, Kim SJ, Kwon HC, Lee DH, et al. Pulmonary marginal zone B-cell lymphoma of MALT type--what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy?: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol. 2010. 89:563–568.
15. Ahmed S, Kussick SJ, Siddiqui AK, Bhuiya TA, Khan A, Sarewitz S, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer. 2004. 40:1320–1326.
16. Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest. 2008. 133:433–440.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download