Abstract
A 47-year old man visited our hospital because of purulent sputum for 3 months. Chest X-ray showed destruction of both the upper lungs, and bronchoscopy revealed inflammatory change with whitish plaque on the left main bronchus through upper division of the left upper lobe. Tracheobronchial aspergillosis (TBA) was finally diagnosed as a result of histologic and microbiologic examination. However, he went abroad without medication before the diagnosis was made and visited again 10 months later. Follow-up bronchoscopy showed complete regression of the previously noted endobronchial lesion. We describe this case to consider the role of antifungal treatment in immunocompetent hosts, as well as to discuss a rare condition; TBA resolved spontaneously.
Aspergillus species is a ubiquitous organism and weak pathogen causing opportunistic infections in immunocompromised hosts. Colonization occurs as a result of inhalation of Aspergillus conidia and if the spore reaches peripheral lung, a variety of clinical syndromes may develop1. Defense mechanisms against Aspergillus infection include bronchial mucociliary reactions, phagocytosis by alveolar macrophages and polymorphonuclear leukocytes, innate T cell response and complement systems2,3.
The spectrum of pulmonary aspergillosis ranges from aspergilloma, chronic necrotizing pulmonary aspergillosis, invasive aspergillosis to allergic bronchopulmonary aspergillosis depending on types of host-fungus relationships.
Tracheobronchial aspergillosis (TBA) is an uncommon clinical form of invasive Aspergillus infection limited to the tracheobronchial tree4. Invasive pulmonary aspergillosis affects mostly immunocompromised hosts such as patients with hematological malignancy with neutropenia and solid organ transplant recipients. It is well known that early diagnosis and initiation of antifungal therapy is important in immunocompromised hosts5. But the role of antifungal therapy in immunocompetent patients has not been defined, because TBA is rare in these hosts. We report a rare case of TBA in an immunocompetent individual, spontaneously regressed without antifungal treatment.
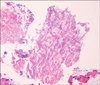
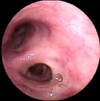
A 47-year-old male presented with purulent sputum for 3 months. He had been diagnosed with pulmonary tuberculosis and prescribed anti-tuberculosis medications two times, for 1 year each, 27 years and 5 years ago. He had no other underlying chronic disease and had not taken any medications that might influence his immune status. He was an ex-smoker with history of 10-pack-years and did not have alcohol abuse history. The patient did not have fever or night sweating and the lungs were clear on auscultation. On chest radiography, there were scar changes in both upper lobes (Figure 1). The white blood cell count was 7,100/mm3 with 63% segmented neutrophils, 24.5% lymphocytes, 7.2% monocytes and 4.6% eosinophils, highly sensitive C-reactive protein was 2.68 mg/dL (reference range, <0.3 mg/dL). The routine blood chemistry tests were normal and enzyme-linked immunosorbent assay of human immunodeficiency virus antibody was negative. Chest computed tomography revealed traction bronchiectasis, fibrous scar and bullous changes in both upper lobes without definite parenchymal infiltrations. On pulmonary function test, forced expiratory volume in 1 second (FEV1) was 1.52 L (42% of predictive value), forced vital capacity (FVC) was 3.06 L (64% of predictive value) and FEV1/FVC was 50%. On flexible bronchoscopy, there were multiple whitish plaques with edematous and inflammatory mucosal changes from mid portion of left main bronchus to upper division of left upper lobe bronchus (Figure 2). Acid-fast bacilli (AFB) stain of bronchial washing and brushing specimen and mucosal biopsy were negative. Hyphae of Aspergillus were seen in mucosal biopsy specimen (Figure 3) and Aspergillus species were cultured from bronchial washing fluid specimen. AFB culture of bronchial washing fluid specimen was negative. Aspergillus Ab IgG was 88 U/mL (reference range, 8~12 U/mL). However, he went abroad without medication before the diagnosis was made and revisited 10 months later. Follow-up bronchoscopy revealed complete regression of the previously noted whitish plaques and inflammatory mucosal changes (Figure 4). Two years later, bronchoscopy was done again and no endobronchial abnormality was found.
TBA was first described in 1991 by Kramer et al.6 as invasive Aspergillus tracheobronchitis after lung transplantation. After that, cases of TBA in immunocompetent hosts have also been reported in chronic constricted airway4, post-tuberculosis tracheal stenosis7, and anastomosis site after lobectomy8. TBA is classified as invasive pulmonary aspergillosis, which is diagnosed based on host factor, clinical criteria and mycological criteria according to the European Organization for Research and Treatment of Cancer/Mycosis Study Group definition9. There were several classifications of TBA from 1991. Most recently, Wu et al.4 classified isolated invasive TBA (iITBA)-TBA without invasive parenchymal disease-according to the bronchoscopic findings findings as superficial infiltration, full-layer involvement, occlusion, and mixed type in 2010. Our patient had mild plaques without airway obstruction or deep tissue invasion, to be classified as superficial infiltration type.
TBA can cause fever, cough, purulent sputum and hemoptysis with or without parenchymal infiltration. These symptoms and signs are nonspecific. Also TBA is not usually associated with parenchymal infiltration in its initial stage, early bronchoscopic evaluation with clinical suspicion is important for early diagnosis5. Prognosis of TBA in immunocompetent hosts is not well known due to its rarity, but that in immunocompromised hosts is poor, mortality rate up to 70%10. Key factors for favorable outcome are an early diagnosis and adequate antifungal therapy10.
Infectious Disease Society of America recommended voriconazole as initial therapy in treatment guidelines for invasive pulmonary aspergillosis including TBA in 20085. But this guideline did not categorized treatment of TBA by host immune status or subtypes of TBA. Wu et al.4 reported that according to the classification of iITBA based on intraluminal lesion, full-layer invasion of the involved bronchus might indicate advanced disease and poor outcome. In our case, intact host immunity and superficial invasion of Aspergillus could explain the spontaneous regression of TBA.
We suggest that TBA in immunocompetent host, especially with superficial bronchial involvement, conservative approach without antifungal agent may be supported by this case of spontaneous regression. There are several case reports11,12 that support our hypothesis. Considering that the types of TBA were different and this is the only one case report, further investigations are needed to establish treatment strategy concerning TBA.
In summary, we experienced a case of TBA resolved spontaneously, suggesting treatment of TBA might be individualized according to the host immune status and the extent of bronchial invasion by Aspergillus.
Figures and Tables
Figure 1
Initial chest X-ray showing old scar of pulmonary tuberculosis without infiltrative lesions in both upper lobes.

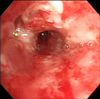
Figure 2
Initial bronchoscopic image showing with edematous, inflammatory changes of bronchial mucosa with whitish plaques.
References
1. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002. 121:1988–1999.
2. Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009. 22:447–465.
3. McCormick A, Loeffler J, Ebel F. Aspergillus fumigatus: contours of an opportunistic human pathogen. Cell Microbiol. 2010. 12:1535–1543.
4. Wu N, Huang Y, Li Q, Bai C, Huang HD, Yao XP. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect. 2010. 16:689–695.
5. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008. 46:327–360.
6. Kramer MR, Denning DW, Marshall SE, Ross DJ, Berry G, Lewiston NJ, et al. Ulcerative tracheobronchitis after lung transplantation: a new form of invasive aspergillosis. Am Rev Respir Dis. 1991. 144(3 Pt 1):552–556.
7. Pornsuriyasak P, Murgu S, Colt H. Pseudomembranous Aspergillus tracheobronchitis superimposed on post-tuberculosis tracheal stenosis. Respirology. 2009. 14:144–147.
8. Garcia-Olivé I, Andreo F, Rosiñol O, Sanz-Santos J, Font A, Monsó E. Bronchial stump aspergillosis after lobectomy for lung cancer as an unusual cause of false positive fluorodeoxyglucose positron emission tomography and computed tomography a case report. J Med Case Rep. 2011. 5:72.
9. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008. 46:1813–1821.
10. Krenke R, Grabczak EM. Tracheobronchial manifestations of Aspergillus infections. ScientificWorldJournal. 2011. 11:2310–2329.
11. Kim JS, Rhee Y, Kang SM, Ko WK, Kim YS, Lee JG, et al. A case of endobronchial aspergilloma. Yonsei Med J. 2000. 41:422–425.
12. Kim TH, Yong BJ, Kim YK, Lee YM, Kim KU, Uh ST, et al. A case of endobronchial aspergilloma with massive hemoptysis. Tuberc Respir Dis. 2004. 57:589–593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download