Abstract
Background
Vitamin D can translocate a vitamin D receptor (VDR) from the nucleus to the cell membranes. The meaning of this translocation is not elucidated in terms of a role in pathogenesis of chronic obstructive pulmonary disease (COPD) till now. VDR deficient mice are prone to develop emphysema, suggesting that abnormal function of VDR might influence a generation of COPD. The blood levels of vitamin D have known to be well correlated with that of lung function in patients with COPD, and smoking is the most important risk factor in development of COPD. This study was performed to investigate whether cigarette smoke extracts (CSE) can inhibit the translocation of VDR and whether mitogen activated protein kinases (MAPKs) are involved in this inhibition.
Methods
Human alveolar basal epithelial cell line (A549) was used in this study. 1,25-(OH2)D3 and/or MAPKs inhibitors and antioxidants were pre-incubated before stimulation with 10% CSE, and then nucleus and microsomal proteins were extracted for a Western blot of VDR.
Results
Five minutes treatment of 1,25-(OH2)D3 induced translocation of VDR from nucleus to microsomes by a dose-dependent manner. CSE inhibited 1,25-(OH2)D3-induced translocation of VDR in both concentrations of 10% and 20%. All MAPKs inhibitors did not suppress the inhibitory effects of CSE on the 1,25-(OH2)D3-induced translocation of VDR. Quercetin suppressed the inhibitory effects of CSE on the 1,25-(OH2)D3-induced translocation of VDR, but not in n-acetylcysteine.
Vitamin D is known to play a wide role in maintaining and extinguishing cells including division, differentiation and apoptosis and there have been a lot of studies of the vitamin D in chronic respiratory diseases1. It is reported that decreasing vitamin D concentration in the bloodstream for adults with bronchial asthma is related to decreasing the forced expiratory volume in 1 second (FEV1) and increasing respiratory tract sensitivity2. It is reported that the concentration of 25(OH)D (25-hydroxyvitamin D) in the bloodstream is closely related with the FEV1 and the forced vital capacity by the U.S. Third National Health and Nutrition Survey (NHANES III)3. Also, Global Initiative on Obstructive Lung Disease (GOLD) 3 or 4 patients have significantly low concentration of 25(OH)D in the blood compared to smokers with normal lungs and this decrease is related to lowering the FEV1 in the study for chronic obstructive pulmonary disease (COPD) patients4. However, some studies report that there is no relation between the 25(OH)D concentration in the blood and the FEV15. Therfore, the relation between the vitamin D concentration and lung functions is not conclusive.
The actions of vitamin D appears through two routes similar to other steroid hormones. First one is through the genomic pathway and this is the transcription process of genomes by stimulating promoters of certain genomes with the vitamin D receptor elements after combining activated vitamin D (1,24-25-D3) and vitamin D receptor (VDR)6. Second one, non-genomic pathway, is the activation process of mitogen activated protein kinases (MAPKs) by combining the VDR on the cell membrane and vitamin D7. It is now known which route is related to the cause of the COPD or involved in the disease process.
The vitamin D in the animal muscle cells increases the VDR translocation from the nucleus to the cytoplasm8 and the proclatin increases the amount of the VDR in the nucleus by translocating the VDR from the cytoplasm to the nucleus. It means that the VDR may translocate from the nucleus to the cytoplasm by chemicals or certain conditions which affect chemicals or cells. Smoking is already known as the most important factor in the COPD but it is now known how the smoking accurately affect the metabolism of vitamin D. Meanwhile, it is reported that smoking decreases 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the bloodstream9. However, there have been no report on the VDR translocation and how the smoking acts at the cell level.
Therefore, the purpose of the study is to examine whether the cigarette smoking extracts inhibits the VDR translocation (from the nucleus to the cytoplasm) by vitamin D in the alveolar basal epithelial cells and if so, how the inhibition happens by which signal transduction.
The strain used in the study was A549, alveolar basal epithelial cell. The cell was cultured in 100 mm plastic dish containing Dulbeco's modified eagle medium (DMEM) with 10% of fetal bovine serum and cultivating in the humidified 5% CO2 cultivator. The plating was performed until the cell confluency reached 70% and the starvation for 18 hours before.
The CSE was manufactured by modifying the method of Smelter et al.10. In brief, 50 mL of syringe was attached to the smoking apparatus (Smoking Tester System; Three Shine Inc, Daejeon, Korea) in the atmosphere. Thirty-five mL of smokes from Korean cigarettes (THIS; KT&G, Seoul, Korea) was slowly inhaled into the attached syringe and passed through 30 mL of cell cultivation DMEM. Each cigarette was used for 10 mL of the DMEM and the smoke passed through 0.2µL micro-filter. The concentration from the process was considered 100% and used within 30 minutes after the manufacturing.
A549 strain with starvation for 18 hours was stimulated with different concentrations and time intervals to investigate the MAPKs activated by the CSE and the western blots of p-C-Jun N-terminal kinase (JNK), pp38, p-extraceullar signal-regulated kinases (ERK) was performed.
First, the proper concentration was determined by treating with the various concentration (1 nM, 10 nM, and 50 nM) of 1,25-(OH2)D3 for 5 minutes to investigate the VDR translocation. Then, to know the inhibition of VDR translocation by CSE, cells were treated with various concentrations (1%, 5%, and 10%) of CSE for 10 minutes and then stimulated for 5 minutes by 1,25-(OH2)D3.
The inhibitors in the study were n-acetylcysteine (NAC; Sigma-Aldrich, St. Louis, MO, USA), PD98059 (Cayman, Ann Arbor, MI, USA), PD169316 (Cayman), and SP600125 (Cayman) and were treated in advance for 1 hour before stimulation with the CSE.
Separating nucleus, cytoplasm, and microsomal protein used NE-PER nuclear and cytoplasmic extraction reagents kit (Thermo Scientific, Rockford, IL, USA) and followed the instruction from the manufacturer. In short, the cultured cells were physically separated by the cell scraper and incubated for 5 minutes at the temperature of 4℃ by adding the cytoplasmic extraction reagents. Then, it was under centrifugation for 5 minutes at 14,000 rpm. Then the supernatant was used to obtain cytoplasm and microsomal protein and the pellet was used to extract nucleus protein. The supernatant was centrifuged for 1 hour at the speed of 100,000 g and the cytoplasm protein and the microsomal protein were in the supernatant and the pellet, respectively. The nuclear extraction reagents was added to the pellet for nucleus protein extract and incubated for 40 minutes at the temperature of 4℃. Then, it was centrifuged for 10 minutes at 14,000 rpm and the supernatant was the nucleus protein.
The used antibodies in the study were anti-JNK (rabbit polyclonal IgG), anti-ERK (rabbit polyclonal IgG), anti p-JNK (mouse monoclonal IgG1), anti p-ERK (rabbit monoclonal IgG) and purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and the anti-VDR antibody (rabbit polyclonal IgG) was purchased from Abcam (Cambridge, UK).
The lysate from the achieved cells was used for the Western blot with the conservative method using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. In short, the nitrocellulose membrane was treated with 5% milk-TBS to eliminate non-specific reactions. Then, it was rinsed with the TTBS (0.05% tween in TBS), reacted with secondary antibodies and rinsed with the TTBS. The coloration used the enhanced chemilumenescence detection kit (Amersham International, Buckinghamshire, UK).
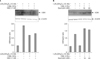
The VDR of microsome more increased by stimulation with 1 nM and 10 nM of 1,25-(OH2)D3 compared to that without stimulation. Ten nM of 1,25-(OH2)D3 showed more translocation than 1 nM, meaning that vitamin D translocate VDR with dose-dependent manner (Figure 1).
1,25-(OH2)D3 of 10 nM was treated in advance for 5 minutes and stimulated with various CSEs to check whether the VDR translocation by vitamin D may be inhibited by the CSE. As shown in Figure 2, the VDR translocation was observed in the microsome without stimulation from the CSE but the VDR translocation significantly inhibited by the treatments of 10% and 20% of CSE.
The strain was stimulated with 1%, 5%, and 10% of the CSE for 30 minutes to investigate which kinase out of the MAPKs was activated by the CSE. As shown in Figure 3, the ERK showed no difference in activation with or without activation of 1% CSE but it showed that the ERK activation significantly increase when stimulated with 5% or 10% of the CSE. However, the ERK was constantly observed for all the cases. However, the JNK and p38 were never activated (data are not shown).
1,25-(OH2)D3 of 10nM was treated in advance for 5 minutes and then the MAPKs inhibitor (PD169316, PD98059, SP600125) was treated and stimulated by the CSE for 30 minutes to investigate the role of the MAPKs. As shown in Figure 4, the VDR translocation was observed by treatment with 1,25-(OH2)D3 as shown in Figure 1 and the translocation was inhibited by the CSE. The translocation inhibition of the VDR by the CSE showed no changes even after treating all the MAPKs inhibitors.
1,25-(OH2)D2 of 10 nM was treated for 5 minutes in advance to investigate whether the antioxidant (NAC and quercetin) prohibits the VDR translocation inhibition of the CSE. Then the NAC and quercetin were treated and stimulated by 10% of CSE for 30 minutes. As shown in Figure 5A, the NAC did not affect the VDR translocation inhibition by the CSE. However, as shown in Figure 5B, quercetin significantly increased the VDR translocation at both 10 nM and 20 nM, meaning that quercetin decreased the VDR translocation inhibition from the CSE.
The study confirmed that the CSE inhibited the VDR translocation. And the signal transmission may decrease by the non-genomic pathway in vitamin D even though the vitamin D had sufficient concentration in the bloodstream in patients with COPD. Of course, the study did not clarify how the CSE inhibited the VDR translocation through a certain signal transmission pathway but it showed that the transmission was not performed by the MAPKs.
It was confirmed that the VDR showed significant translocation depending on vitamin D concentrations as shown in Figure 1 when stimulated by vitamin D to check whether the VDR showed translocation to the microsome by vitamin D. The result coincided with that of Capiati et al.8 and the VDR translocation by vitamin D was proved. Based on the result, vitamin D is used for the VDR translocation on the following experiments in this study.
Up to now, there has been no report that the VDR translocation was inhibited by CSE and was confirmed by this study for the first time. As shown in Figure 2, the VDR translocations by vitamin D significantly decrease at the concentrations of 10% and 20% of the CSE. Up to now, it is unknown how the VDR translocation affected lungs. However, it was observed that the VDR protein significantly decreased in the lungs of patients with COPD compared to in the lungs of smokers, the matrix metalloproteinases increase was observed for VDR knock-out mice and caused the emphysema, suggesting that the VDR played a crucial role in the COPD11. However, this experiment inhibited both pathways and does not know which pathway is related to the emphysema.
Of course, decrease of the VDR differs from isolation of the VDR inside the nucleus. However,it is clear that decreasing signal transmission by the VDR is related to the COPD. Up to now, it has shown that the genetic expressions of osteocalcin and osteopontin increased through the signal transmission by genomic pathway of vitamin D, interleukin (IL)-2 and IL-12 or inflammatory genome expressions decreased12 and the MAPKs were activated through the non-genomic pathway even though it is not clear7. However, furtherstudies are needed because it is not clear how these study results play a role in generating and preventing the COPD through signal transmission pathway of the VDR inside the cell membrane and nucleus.
The concentrations of the CSE in the study may be different from the exposure of alveolar epithelial cells in smokers' lungs to the smoking. The CSE concentrations shall be measured from smokers' lungs and the figures shall be used in the study for the study to have clinical meanings but there has been no report on measuring the CSE concentrations from smokers' lungs. However, there are several reports measuring nicotine concentrations from bloodstream rather than from smokers' lungs13 and other studies measure the concentrations in the respiratory tract after the exposure to smoking for in vitro and in vivo14. The CSE consists of many chemical compounds and it is impossible to estimate the CSE concentration from the nicotine concentration. Therefore, we inevitably used 10% of CSE based on the dose-dependent study.
The study of Shen et al.15 confirmed that the ERK was activated in the respiratory tract epithelial cells by the CSE like the results of this. Therefore, the authors predicted that inhibiting the activated MAPKs activated by the CSE may prevent the inhibition of the VDR translocation from the CSE, however, all the MAPKs inhibitors could not reverse the inhibition of VDR translocation as shown in Figure 4. In particular, the ERK is activated by the CSE and it is expected that the inhibition of ERK may reproduce the VDR translocation by vitamin D. Of course, it is impossible to decide the VDR translocation was performed through MAPK pathway, although simple treatment with MAPKs inhibitor reverse the VDR translocation. This study does not accurately know which pathway is related. However, there were no changes in the SMAD3 and the SMAD4 when the CSE stimulated A549 cells or respiratory tract epithelial cells but the SMAD6 and the SMAD7, inhibiting SMADs, decreased16, meaning that the CSE was through the SMAD signal transmission system by the TGF-beta was not ruled out. Another pathway, Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, was not ruled out because the NADPH oxidase was activated when the CSE stimulated endothelial cells and then the JAK/STAT pathway was activated17. Additional studies on the signal transmission processes are required in the future.
Quercetin is flavonoids much contained in vegetables
or tea18 and is known to have inhibition effects on athergenic, hypertension and obesity19-21. Also, the action is known due to strong antioxidant. The reason of using quercetin in the study is that quercetin increases the target genome of the VDR, TRPV6 mRNA and it is because quercetin increases the VDR activation22. Unlike quercetin, n-acetylcysteine used in the study may not inhibit the VDR translocation from the CSE. This result suggests that the antioxidant itself may not inhibit the functions of the CSE and the CSE may be inhibited only if it is related to the genomic transcription of the VDR.
In conclusion, it was confirmed that the VDR translocation was inhibited by the CSE and this inhibition had different pathway from the MAPKs signal pathway.
Figures and Tables
Figure 1
Effects of 1,25-(OH)2D3 on the subcellular distribution of vitamin D receptor (VDR) in A549 cells. Proteins from microsomal and nuclear fractions from A549 cells treated for 5 min with 1 nM and 10 nM 1,25-(OH)2D3 were subjected to Western blot for anti-VDR antibody. Microsomal fractions of VDR are more increased by treatment with 1,25-(OH)2D3, compared to the control. Results of densitometric analysis are shown in the lower histogram.

Figure 2
Effects of cigarette smoke extracts (CSE) on the subcellular distribution of vitamin D receptor (VDR) in A549 cells. Proteins from microsomal and nuclear fractions from A549 cells treated for 10 min with 10% and 20% of CSE after pre-incubation of 5 min with 10 nM 1,25-(OH)2D3 were subjected to Western blot for anti-VDR antibody. Both 10% and 20% of CSE inhibit the 1,25-(OH)2D3-induced translocation of VDR. Results of densitometric analysis are shown in the lower histogram.

Figure 3
Effects of cigarette smoking extracts (CSE) on the activation of extraceullar signal-regulated kinases (ERK) in A549 cells. Proteins from A549 cells treated for 30 min with 1%, 5%, and 10% of CSE were subjected to Western blot for anti-p-ERK and anti-ERK antibody. ERK are activated with CSE treatment by dose-dependent pattern. Results of densitometric analysis are shown in the lower histogram.

Figure 4
Effects of mitogen activated protein kinases (MAPKs) on the 1,25-(OH)2D3-induced translocation of vitamin D receptor (VDR) in the presence of cigarette smoking extracts (CSE) in the A549 cells. Proteins from microsomal fractions from the A549 cells treated for 1 hr of MAPKs inhibitors before 10% of CSE treatment were subjected to Western blot for anti-VDR antibody. All MAPKs inhibitors cannot reverse the inhibitory effects of CSE on the 1,25-(OH)2D3-induced translocation of VDR. Results of densitometric analysis are shown in the lower histogram.

Figure 5
Effects of anti-oxidants on inhibitory effects of cigarette smoking extracts (CSE) on the1,25-(OH)2D3-induced translocation of vitamin D receptor (VDR) in the A549 cells. Proteins from microsomal fractions from the A549 cells treated for 1 hr of n-acetylcystein (NAC) (A) and quercetin (B) before 10% of CSE treatment were subjected to Western blot for anti-VDR antibody. Quercetin reversed the inhibitory effects of CSE on the 1,25-(OH)2D3-induced translocation of VDR, but NAC did not. Results of densitometric analysis are shown in the lower histogram.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090548).
References
1. Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009. 158:20–25.
2. Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010. 181:699–704.
3. Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005. 128:3792–3798.
4. Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010. 65:215–220.
5. Shaheen SO, Jameson KA, Robinson SM, Boucher BJ, Syddall HE, Sayer AA, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011. 66:692–698.
6. Ting HJ, Bao BY, Reeder JE, Messing EM, Lee YF. Increased expression of corepressors in aggressive androgen-independent prostate cancer cells results in loss of 1alpha,25-dihydroxyvitamin D3 responsiveness. Mol Cancer Res. 2007. 5:967–980.
7. Buitrago C, Boland R. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010. 121:169–175.
8. Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002. 86:128–135.
9. Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999. 53:920–926.
10. Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010. 185:3035–3040.
11. Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011. 406:127–133.
12. Gallieni M, Cozzolino M, Fallabrino G, Pasho S, Olivi L, Brancaccio D. Vitamin D: physiology and pathophysiology. Int J Artif Organs. 2009. 32:87–94.
13. Bernert JT, Jacob P 3rd, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009. 11:1458–1466.
14. Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol. 2008. 32:201–207.
15. Shen N, Gong T, Wang JD, Meng FL, Qiao L, Yang RL, et al. Cigarette smoke-induced pulmonary inflammatory responses are mediated by EGR-1/GGPPS/MAPK signaling. Am J Pathol. 2011. 178:110–118.
16. Springer J, Scholz FR, Peiser C, Groneberg DA, Fischer A. SMAD-signaling in chronic obstructive pulmonary disease: transcriptional down-regulation of inhibitory SMAD 6 and 7 by cigarette smoke. Biol Chem. 2004. 385:649–653.
17. Shih RH, Lee IT, Hsieh HL, Kou YR, Yang CM. Cigarette smoke extract induces HO-1 expression in mouse cerebral vascular endothelial cells: involvement of c-Src/NADPH oxidase/PDGFR/JAK2/STAT3 pathway. J Cell Physiol. 2010. 225:741–750.
18. Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008. 11:733–740.
19. Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, Moreno L, et al. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun. 2006. 346:919–925.
20. Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007. 137:2405–2411.
21. Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring). 2008. 16:2081–2087.
22. Inoue J, Choi JM, Yoshidomi T, Yashiro T, Sato R. Quercetin enhances VDR activity, leading to stimulation of its target gene expression in Caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 2010. 56:326–330.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download