Abstract
Background
Oxidation plays an important role in acute lung injury. This study was conducted in order to elucidate the effect of repetitive post-treatment of N-acetylcysteine (NAC) in lipopolysaccaride (LPS)-induced acute lung injury (ALI) of rats.
Methods
Six-week-old male Sprague-Dawley rats were divided into 4 groups. LPS (Escherichia coli 5 mg/kg) was administered intravenously via the tail vein. NAC (20 mg/kg) was injected intraperitoneally 3, 6, and 12 hours after LPS injection. Broncho-alveolar lavage fluid (BALF) and lung tissues were obtained to evaluate the ALI at 24 hours after LPS injection. The concentration of tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were measured in BALF. Nuclear factor κB (NF-κB), lipid peroxidation (LPO), and myeloperoxidase (MPO) were measured using lung tissues. Micro-computed tomography (micro-CT) images were examined in each group at 72 hours apart from the main experiments in order to observe the delayed effects of NAC.
Results
TNF-α and IL-1β concentration in BALF were not different between LPS and NAC treatment groups. The concentration of LPO in NAC treatment group was significantly lower than that of LPS group (5.5±2.8 nmol/mL vs. 16.5±1.6 nmol/mL) (p=0.001). The activity of MPO in NAC treatment group was significantly lower than that of LPS group (6.4±1.8 unit/g vs. 11.2±6.3 unit/g, tissue) (p<0.048). The concentration of NF-κB in NAC treatment group was significantly lower than that of LPS group (0.3±0.1 ng/µL vs. 0.4±0.2 ng/µL) (p=0.0001). Micro-CT showed less extent of lung injury in NAC treatment than LPS group.
The acute respiratory distress syndrome (ARDS), progressed from the acute lung injury (ALI) is a critical disease with high prevalence rates and mortality in serious patients1. The sepsis, the most frequent cause of the ALI, is mediated by the lipopolysaccharide (LPS). The sepsis appears in 18~42% of patients with Gram-negative bacterial infection and is developed to the ARDS with high mortality of about 50%2. By injecting LPS into vein or trachea in the animal model, infiltration of the neutrophil into the lung tissues increases. Release of various proinflammatory cytokine and the reactive oxygen species (ROS) from these neutrophils into the lung and blood play a crucial role in provoking the tissue damage3-6.
The toxic oxygen species are also produced by macrophages apart from activated neutrophils and cause damages to endothelial cell in the lung. The toxic oxygen species change cell functions and structures by reaction with lipid, protein, nucleic acid, and carbohydrate in the cells, and caused peripheral organ failures through a series of processes with various inflammatory mediators5,7,8.
There are anti-oxidants and against oxidants in the human body but most of them are consumed during the acute inflammatory reaction and the excess ROS is produced from neutrophils and macrophages during the ALI causes the cell injury. Excess ROS can also be produced by high concentration of oxygen which is often provided by the mechanical ventilation to patient with ARDS2,9 through the reaction with the NADPH oxidase on the cell membrane of the neutrophil infiltrated into the lung and consequently increase the oxidation stress and exacerbate tissue damages10.
N-Acetylcysteine (NAC) is a thiol compound with the sulfhydryl group and precursor of glutathione, direct ROS scavenger and regulates the redox reaction which modulates the gene expression and inflammatory reaction in cell11. The NAC did not improve PaO2/FiO2 or reduce the mortality among patients with ARDS. However, it has been reported that the NAC reduced fibrin in lung tissue, increased glutathione in the erythrocytes and shortened the period of morbidity of the ARDS12,13.
Although it has been known that the exogenous anti-oxidants are effective in the animal model, the efficacy is unclear, because the anti-oxidant has a short half-life and is difficult to penetrate into the cell membranes due to a large molecules14-16. It was recently reported that single injection of the liposomal-NAC which was used to enhance the absorption rates was effective in the LPS-induced ALI in the animal studies17. However, the effects of repeated injection of the NAC without liposome after causing lung damages are not clear yet.
Thus this study is aimed to investigate the therapeutic effect and its related intracellular mechanism(s) of repeated administrations of NAC into rats after induction of ALI by systemic LPS injection.
A total of 48 male pathogen-free Sprague-Dawley rats (Biolink, Eumseong, Korea) with 6 weeks old were used for the experiment.
The animals were divided into 4 groups with 12 rats each for saline control (SC), NAC control (NC), LPS and NAC treatment (NACTX) groups. Because, in the preliminary experiment, most of the rats (80%) were dead within 24 hours after 10 mg/kg of LPS (0.3 mL) (Escherichia coli 0111:B4; Sigma, St. Louis, MO, USA) was used to cause the ALI, 0.3 mL of 5 mg/kg of LPS was injected to LPS and NACTX groups the rat vein after performing inhalation anesthesia with ether. The same amount of normal saline (0.3 mL) was injected to SC and NC groups instead of LPS. Repeated intraperitoneal injection of NAC (Sigma) to LPS group were performed in 3 times at regular intervals, because injection of NAC below or above 3 times were not effective in reducing the lipid peroxidation (LPO) and the nuclear factor kB (NF-κB) in preliminary experiments. Normal saline (0.5 mL) was injected to SC and LPS groups at 3, 6, and 12 hours after the LPS injection. NAC (20 mg/kg, 0.5 mL) was injected intraperitonealy at the same time intervals to NC and NACTX groups.
Pentothal sodium (70 mg/kg, 0.6 mL) was injected via the tail vein after ether anesthesia 24 hours later fallowing LPS injection into the experimental rats. The skin was incised from the abdomen to the head, and blood was taken from the abdominal aorta. The chest was opened to expose the lungs and bronchi. The intratracheal cannula was inserted and, the left main bronchus was tied. The broncho-alveolar lavage (BAL) was performed through the right lung with 6 mL phosphate-buffered saline in 3 times. The total number of cells in BALF was counted with the coulter count. BALF (5 mL) was centrifuged for 10 minutes at 900 rpm by Cytospin (Shandon Co., Pittburgh, PA, USA) and stained with Diff-Quick (modified Giemsa) to calculate the partial number of cells by counting 500 cells at 400 times magnification on the light microscope. The remaining BALF was centrifuged at 400 ×g for 10 minutes and the supernatants were stored at -80℃. The left and the right lungs were enucleated. The remaining blood in the right lung was removed by irrigation (0.9% of ice-cold saline) through the main bronchus. The both lungs were weighed after the BAL as an indicator for the pulmonary edema in the ALI18. The left lung was homogenized with the homogenizer using 50 mM potassium phosphate buffer of pH 7.4. Formalin was injected into the right lung tissue and the lung was stored in the formalin-filled tube to investigate the degree of the ALI using the light microscope. The section of lung tissue were randomly cut and fixed.
The protein contents in BALF were measured by the Brown method after performing the BAL19.
The MPO activity was measured by the specific enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) using crushed left lung to investigate neutrophil activation and infiltration in the lung.
The tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were measured in the BALF by the ELISA kit (R&D Systems).
One hundred mg of the crushed lung tissues was extracted and the LPO concentration was measured by the thiobarbituric acid reactive substances assay kit (Zeptometrix, Buffalo, NY, USA) to investigate the oxidation stress. About 0.5 mg of the nuclear protein (107 cells) was extracted by the Nuclear Extract kit (Active Motif, Shinjuku, Japan) using lung tissues and the NF-κB measured by the TransAM NF-κB p65 transcriptional factor assay kit (Active Motif) to investigate the effect on the signal transmission system of inflammation/immune reactions.
The right lung stored in the formalin were randomly cut and fixed. The alcohol concentration on fixed lung tissues was gradually increased for the dehydration and the remaining alcohol was eliminated by xylene. Then, it was covered with paraffin and sectioned samples with 4µm thickness were prepared, stained by hematoxylin and eosin and observed by the light microscope.
The rats were divided into 4 groups with allotment of 5 animals for each group. The micro-CT (SkySkan 1172 high-resolution micro-CT; SkyScan, Kotich, Belgium) images of extracted lungs in the supine position were taken at 72 hours after the LPS injection. Before imaging, the rats were anesthetized and blood was removed from the heart. Then, the lung was irrigated using 0.9% of ice-cold saline. After 6~8 mL of air was injected into the lung, both main bronchus were tied and the micro-CT was imaged.
The statistical analyses were performed using the SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). The comparisons among SC, NC, LPS, and NACTX groups were made using Kruskal-Wallis test. The comparisons between two groups were made using Mann-Whitney U test. The results are presented as the mean±SE. p<0.05 was considered statistically significant.
Weights of rats measured at the beginning of experiments and at 24 hours after injection of normal saline for SC (from 293.6±16.9 g to 295.8±15.3 g) and NAC for NC groups (from 291.3±12.9 g to 293.3±13.5 g) were not different. However, weights measured at the same time points of experiments were significantly different both in LPS (from 280.3±13.9 g to 262.7±9.3 g) and in NACTX groups (from 291.4±6.9 g to 276.5±6.4 g) (p=0.001) (Table 1). Comparing weight changes among experimental groups at 24 hours after the LPS injection, LPS (p=0.001) and NACTX groups (p=0.001) showed significant weight decrease compared to NAC group but no significant difference in LPS and NACTX groups. The lung weight (g) was significantly low in NACTX group compared to LPS group (1.5±0.2 vs. 1.7±0.2) (p=0.002). The ratio of the lung weight to the body weight was significantly high in LPS group (0.6±0.1) compared to SC (0.5±0.0), NC (0.4±0.1) (p=0.001), and NACTX (0.5±0.1) (p=0.004) groups (Table 1).
The total number of cells in the BALF (×105/mL) increased in LPS (5.8±1.2) and NACTX (4.9±1.1) groups compared to both control, SC (1.5±0.5) and NC (1.4±0.5) (p=0.001) groups. There was no difference between LPS and NACTX groups (p=0.078). The fractions of neutrophil (%) were increased in LPS and NACTX (49.1±5.9, 48.6±6.9) compared to SC and NC groups (1.5±0.9, 1.2±0.8) (p=0.001). There was no difference between LPS and NACTX groups. The protein concentrations (µg/mL) increased in LPS and NACTX groups (72.6±10.2, 66.3±5.9) compared to SC and NC groups (34.5±4.5, 38.3±5.7) (p=0.001), but there is no difference between LPS and NACTX groups (Table 1).
The TNF-α (pg/mL) significantly increased in LPS group compared to both control groups (SC and NC) in BALF (21.8±5.8 vs. 4.5± 2.3, 3.0±2.4) (p=0.0001) but no difference was seen compared with the NACTX groups (23.4±9.8). In addition, the IL-1β (pg/mL) significantly increased in LPS compared to two control (SC and NC) groups in the BALF (150.3±37.2 vs. 10.5±10.6, 7.5±7.9) (p=0.0001). There was no difference as compared with NACTX groups (138.7±21.6) (Table 2).
LPO concentration (nmol/mL) in LPS group was higher compared to SC and NC groups (16.5±1.6 vs. 4.3±3.8, 3.5±2.0) (p=0.0001) but significantly lower when comparing with NACTX group (5.5±2.8) (p=0.001) (Table 3, Figure 1A). The MPO activity in the left lung tissues (unit/g, lung tissue) significantly decreased in NACTX compared to LPS group (6.4±1.8 vs. 11.2±6.3) (p=0.048) (Table 3). LPS group (0.4±0.2) showed the highest NF-κB concentration (ng/µL) compared to SC (0.1±0.0), and NC (0.11±0.0) groups (p=0.0001) and it decreased significantly compared to NACTX group (0.3±0.1) (p=0.006) (Table 3, Figure 1B).
The lung tissues taken from right side were observed as a whole by sagittal section without dividing into the individual lobe. Although there were increased exudates and inflammatory cells in the alveolar space in LPS group, because no much difference was observed in interstitial edema or bleeding in the alveolar space between LPS and NACTX groups, it was considered that there was no pathological difference between two groups by pathologist.
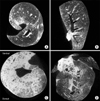
Three rats of LPS group (n=5) and 1 rat of NACTX group (n=5) were expired before taking the micro-CT mages at 72 hours after lung injury. Although the findings were not homogenous in the lung parenchyma, more dominant ground glass opacities were observed in LPS group compared to NACTX group (Figure 2).
In this study, after induction of ALI with LPS, NAC was repeatedly injected to the animals in a similar situation in treating patients with ARDS in the clinical practice. A single injecting dose of NAC for a rat in this experiment is equivalent to a high dose of 1,000 mg in a human. The study showed that the LPO concentration significantly decreased in NACTX group compared to LPS group (7.5±1.6 vs. 15.1±1.1) (p<0.05), as well as the MPO activity (6.4±0.5 vs. 11.2±1.8) (p<0.05) (Table 3). After 72 hours, micro-CT examination for lung injury showed partial improvement of lung injury in NACTX group, suggesting that the NAC decreased the oxidation stress and partially alleviated the degree of the ALI. NF-κB also significantly decreased in NACTX group, suggesting that the NAC inhibited the NF-κB activation and decreased the ROS production.
However, the concentration of TNF-α and IL-1β in BALF had no significant difference between NACTX and LPS groups at 24 hours after the LPS injection and the finding of light microscope on the lung tissue at 24 hours after the LPS injection had also no significant difference between LPS and NACTX groups. These results suggested that later NAC injection after induction of ALI could not decrease previous-formed cytokine and already increased permeability of damaged lung immediately which was developed before NAC administration.
TNF-α and IL-1β are core materials which mediate the initial inflammatory reaction by neutrophil chemotaxis and activation20. Because there were no difference of TNF-α and IL-1β in BALF between LPS and NACTX groups in this experiment, it could be explained why neutrophil infiltration was not decreased in NACTX group. We assumed the reasons why the cytokines were not inhibited by NAC injection. Initial administration of NAC at 3 hours later LPS injection and through intraperitoneal route like our experiment might cause slow rise in concentration of NAC which was not enough to inhibit the initial inflammatory reaction materials such as TNF-α and IL-1β which are reaching the peat level at 3 to 6 hours after initial injury21-23.
In this study, the ALI model was provided by injecting the LPS via vein. The ALI caused by direct lung insult showed two or three times rise of IL-6, IL-8 and IL-10 compared to the ALI due to extrapulmonary systemic diseases24. Therefore, the ALI model induced by LPS injection via vein shows slower inflammatory reaction compared to LPS administration directly into the trachea. This suggests that according to the different model of ALI, the effect of NAC can be different in degree and in time patterns. Inflammatory cells, protein contents and cytokines in BALF of LPS and NACTX groups did not show significant differences at 24 hours after the LPS injection. However, MPO activity and NF-κB concentrations significantly decreased in NACTX compared to LPS group, suggesting that the effect of NACTX might be present in some parts of inflammatory process and incomplete for flawless treatment.
In this study, NF-κB showed significant differences in LPS and NACTX groups, suggesting that the NAC directly affected transcription factors. Therefore, the effect of post-treatment NAC might have been mainly contributed to inhibition of inflammation by reducing ROS through inhibiting NF-κB activation, rather than affecting initial cytokine release from neutrophil infilteration and activation. NF-κB plays a important role in the inflammation process by transcribing cytokine genes as a transcription factor related to oxidation and reduction. Previous studies have reported that upregulation of anti-oxidation genes25 followed by increased generations of anti-oxidant contributed to decreasing inflammation by inhibiting NF-κB18,25-27. The results of this study are consistent with them.
The lung injury in the late process of ALI shows the same histological findings regardless of various causes but has pathophysiologic differences in the early ALI according to causes (extrapulmonary or pulmonary)28. For example, LPS injection via vein distinctly increases NF-κB after 10 hours but the intratracheal LPS injection reached the peak of NF-κB at 6 hours later and rapidly decreased to the normal level at 12 hours later29,30. Therefore, in induced ALI by LPS injection via vein, repetitive NACTXs can be more effective in reducing NF-κB. There were no change in the initial inflammatory cytokines but the MPO activity mainly generated by neutrophil was significantly decreased in NACTX than LPS group, suggesting that the NAC decreased activation of neutrophil.
In addition, this study showed that the NAC inhibited LPO production. LPO in the lung tissue was reported to increase along with the ROS production in the animal study31. ROS in the lung was generated in macrophages, neutrophil, endothelial and epithelial cells31. The LPO is related to severe inflammatory diseases and plays a potential mechanism in tissue damages. LPO is an indicator for oxidation stress often expressed by the level of malondialdehyde and one the major mechanism of tissue damage2,5,32. In the process of the acute inflammatory reaction, anti-oxidants (glutathione, superoxide dismutase, catalase, and glutathione peroxidase) against the oxidation stress rapidly decrease in the human body and the NAC injection is known to inhibit the toxic effect of the ROS. Repeatedly injected NAC complements depleted anti-oxidants in the human body and shows effects by increasing non-protein thiols17,25,26,33. Although there was a report that the NAC injection through oral cavity or vein did not significantly increase anti-oxidant concentration or metabolites in lung tissues34, on the contrary, the other reported that the cysteine concentration in the human body elevated after injection of NAC to patients with ARDS13. Other studies have reported that it has protection effects when high concentration of NAC (150 mg/kg/hr) is continuously injected via vein before injecting the LPS (10 mg/kg)16. The present study showed that NAC administration also had a post-treatment effect. A recent study reported single injection of NAC with increased half-life using liposome could diminish lung inflammation and ROS production by carrying more anti-oxidants through prolonged accumulation in injured lung tissue due to the LPS17. In the present study, repetitive injections of ordinary NAC also showed similar anti-oxidant effect to the single injection of liposomalized NAC evidenced by decreased LPO activity.
In the preliminary experiment, injection of NAC>3 times were not effective on reducing LPO and NF-κB concentrations. There was a similar report to our experiment that continuous injection of the NAC for therapeutic purpose through vein in pig model did not increase the glutathione concentration and no treatment effect was seen35. Although there was no clear explanation for this phenomenon, it may be important in balance between substrates and enzymes in generating glutathione.
The findings of light microscope in LPS and NACTX groups at 24 hours after LPS injection did not show significant differences in the extent of the infiltration of inflammatory cells. However, 3 days later inducing the ALI, the micro-CT findings in vitro showed that ground glass opacity was definitely decreased in NACTX group compared to LPS group, suggesting that slowly progressive improvement of inflammation in NACTX group. Thus it was considered that NAC had delayed effects with time evidenced by the micro-CT findings taken at 72 hours later.
This study had several limitations. First, the concentration NAC and anti-oxidants were not directly measured in the lung or blood. Second, the cytokine may not be measured at the peak period. Third, the micro-CT and pathologic findings at 72 hours were not compared each other. Nevertheless, the present study showed repetitive injections of NAC in a post-treatment manner had partial but significant therapeutic effects by increasing anti-oxidation effect on ALI in a relatively later stage.
This study evaluated treatment effect of repetitive injection of NAC after inducing ALI to coincide with the actual clinical fields, rather than injecting the LPS for a preventive purpose performed in most studies. Later administration of NAC after induction of ALI had no effects on previous-formed cytokine and already increased permeability of damaged lung just immediately after NAC injection. However worsening of ALI which was inevitable in the course of untreated ALI with time was alleviated. There were partial but significant therapeutic effects on ALI in a relatively later stage.
The suggested mechanisms of post-treatment effect of NAC were mainly due to inhibition of inflammation by reducing ROS through inhibiting NF-κB activation, rather than affecting initial cytokine release from neutrophil infilteration and activation.
Figures and Tables
Figure 1
The effects of N-acetylcysteine (NAC) of nuclear factor κB (NF-κB) and lipid peroxidation in lipopolysaccharide (LPS) induced acute lung injury. (A) Lipid peroxidation concentrations in lung tissue. In NAC treatment (LPS+NAC) group, lipid peroxidation concentration significantly decreased than that in LPS group. (B) NF-κB concentrations in lung tissue. NF-κB concentration in NAC treatment group significantly decreased more than that in LPS group. Box table: median (25~75%); except out-layer data.

Figure 2
Micro-computed tomography (micro-CT) of rat model. The N-acetylcysteine (NAC) group (A, right lung; B, left lung) shows normal lung parenchyma in micro-CT. The lipopolysaccharide (LPS) group (C) and NAC treatment group (NAC+LPS group) (D) show ground glass opacity pattern in Rt. lower lobe, but in NAC treatment (D), the ground glass opacity decreased more than that in LPS group (C).
References
1. Costa EL, Schettino IA, Schettino GP. The lung in sepsis: guilty or innocent? Endocr Metab Immune Disord Drug Targets. 2006. 6:213–216.
2. Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004. 202:145–156.
3. Koh Y, Lee YM, Lim CM, Lee SS, Shim TS, Lee SD, et al. Effects of heat pretreatment on histopathology, cytokine production, and surfactant in endotoxin-induced acute lung injury. Inflammation. 2001. 25:187–196.
4. Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989. 320:365–376.
5. Zhang H, Slutsky AS, Vincent JL. Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Med. 2000. 26:474–476.
6. Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003. 29:427–431.
7. Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999. 25:180–185.
8. Ferruzza S, Scarino ML, Gambling L, Natella F, Sambuy Y. Biphasic effect of iron on human intestinal Caco-2 cells: early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol Biol (Noisy-le-grand). 2003. 49:89–99.
9. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007. 369:1553–1564.
10. Dana R, Malech HL, Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994. 297(Pt 1):217–223.
11. Atkinson MC. The use of N-acetylcysteine in intensive care. Crit Care Resusc. 2002. 4:21–27.
12. Jepsen S, Herlevsen P, Knudsen P, Bud MI, Klausen NO. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med. 1992. 20:918–923.
13. Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, et al. The Antioxidant in ARDS Study Group. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. Chest. 1997. 112:164–172.
14. Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001. 71:1–21.
15. Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006. 5:47–78.
16. Kao SJ, Wang D, Lin HI, Chen HI. N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin Exp Pharmacol Physiol. 2006. 33:33–40.
17. Mitsopoulos P, Omri A, Alipour M, Vermeulen N, Smith MG, Suntres ZE. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int J Pharm. 2008. 363:106–111.
18. Kim BY, Lee YM. Moxifloxacin ameliorates oleic acid-induced acute lung injury by modulation of neutrophilic oxidative stress in rats. Tuberc Respir Dis. 2010. 68:334–344.
19. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989. 180:136–139.
20. Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006. 79:1348–1356.
21. Lima Trajano ET, Sternberg C, Caetano M, Santos Silva MA, Porto LC, Santos JC, et al. Endotoxin-induced acute lung injury is dependent upon oxidative response. Inhal Toxicol. 2011. 23:918–926.
22. Kim JH, Yoon DW, Jung KH, Kim HO, Ha ES, Lee KJ, et al. The effects of nuclear factor-kappa B decoy oligodeoxynucleotide on lipopolysaccharide-induced direct acute lung injury. Tuberc Respir Dis. 2009. 67:95–104.
23. Yoshida M, Yoshimura N, Hangai M, Tanihara H, Honda Y. Interleukin-1 alpha, interleukin-1 beta, and tumor necrosis factor gene expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1994. 35:1107–1113.
24. Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005. 98:1777–1783.
25. Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003. 90:221–232.
26. Cadenas S, Cadenas AM. Fighting the stranger-antioxidant protection against endotoxin toxicity. Toxicology. 2002. 180:45–63.
27. Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999. 100:1330–1337.
28. Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003. 42:48s–56s.
29. Matsuda N, Hattori Y, Jesmin S, Gando S. Nuclear factor-kappaB decoy oligodeoxynucleotides prevent acute lung injury in mice with cecal ligation and puncture-induced sepsis. Mol Pharmacol. 2005. 67:1018–1025.
30. Matsuda N, Hattori Y, Takahashi Y, Nishihira J, Jesmin S, Kobayashi M, et al. Therapeutic effect of in vivo transfection of transcription factor decoy to NF-kappaB on septic lung in mice. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1248–L1255.
31. Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008. 10:739–753.
32. Rahman I. Antioxidant therapies in COPD. Int J Chron Obstruct Pulmon Dis. 2006. 1:15–29.
33. Victor VM, Rocha M, De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004. 4:327–347.
34. Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007. 20:9–22.
35. Vassilev D, Hauser B, Bracht H, Iványi Z, Schoaff M, Asfar P, et al. Systemic, pulmonary, and hepatosplanchnic effects of N-acetylcysteine during long-term porcine endotoxemia. Crit Care Med. 2004. 32:525–532.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download