Abstract
Streptococcus suis causes meningitis and sepsis in pigs, but human infection has increased over the past few years in those who are exposed to pigs or raw pork. Most cases have occurred in Southeast Asia, but only two cases have been reported in South Korea, presenting with arthritis and meningitis. Here, we report a rare case of S. suis infection, a 60-year-old sailor, who visited the emergency room presenting septicemia, pneumonia with empyema and meningitis, showed full recovery; however, neurologic sequale of severe cognitive dysfunction was present after the usage of antibiotics and percutaneous drainage. S. suis was isolated from blood and pleural fluid and the strain was susceptible to penicillin and vancomycin. Increased awareness of S. suis infection and prevention are warranted.
Streptococcus suis is a swine pathogen that causes meningitis, sepsis, pneumonia and endocarditis1. S. suis infection was first reported in pigs in 1954 and the first human case was reported in Denmark in 19682. Human infection with S. suis has significantly increased over the past few years. Meningitis is the most common presentation of infection followed by sepsis which has a higher mortality rate, particularly in spleenectomized patients. Other uncommon clinical presentations include enteritis, arthritis, endocarditis, pneumonia, uveitis and peritonitis. The majority of cases have occurred in Southeast Asia but only two cases were reported in South Korea3,4, each case presenting septic arthritis and meningitis respectively. We report a rare case of S. suis infection that occurred in South Korea which caused pneumonia and empyema.
A 60-year-old male presented to the emergency room complaining of fever and coughing that lasted for 2 days. He was working as a sailor around the southern coast of Korea and due to the uncooperative nature of the patient, obtaining a detailed medical history was difficult. His captain said that he drank alcohol every day and he was the only one of his crew with symptoms.
Neurological examination revealed disorientation with no focal findings. The patient scored a 9 on the Glasgow coma scale. His temperature was 37.5℃, blood pressure was 160/98 mm Hg, pulse rate was 96 beats per min, respiratory rate was 22 breaths per min and oxygen saturation was 95% at room air. Lung auscultation revealed coarse crackles in the right whole lung fields.
Complete blood cell count showed a white blood cell count of 11.97×103/µL (normal, 3.9~11.0×103/µL), a hemoglobin concentration of 12.8 g/dL (normal, 13~18 g/dL), and a platelet count 155×103/µL (normal, 140~440×103/µL). The differential count showed neutrophils at 96.4%, lymphocytes at 2.7%. The C-reactive protein level was 38.5 mg/dL (normal, ≤0.3 mg/dL). His blood urea nitrogen was 51 mg/dL (normal, 8~20 mg/dL), creatinine was 1.31 mg/dL (normal, 0.6~1.2 mg/dL), albumin was 2.6 g/dL (normal, 3.8~5.1 g/dL), alkaline phosphatase was 200 U/L (normal, 3~120 U/L), aspartate aminotransferase was 43 IU/L (normal, 7~38 IU/L) and alanine aminotransferase was 21 IU/L (normal, 4~43 IU/L). In the arterial blood gas analysis, PaO2 was 72.2 mm Hg, PaCO2 was 24.6 mm Hg and oxygen saturation was 95%.
Pleural fluid examination showed pH 7.0, glucose 2 mg/dL, protein 4.0 g/dL, white blood cell 137,000/mm3 (polymorphonuclear leukocyte 95%, lymphocyte 5%).
Both blood and pleural fluid cultures grew S. suis which was identified by using the automated Vitek2 system. Antimicrobial susceptibility testing using disc diffusion method showed that the isolate was sensitive to penicillin, erythromycin, clindamycin, cefepime and vancomycin but resistant to tetracycline (Table 1).
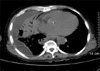
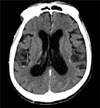
The initial chest X-ray showed diffuse hazy opacity on the right upper and lower portion (Figure 1). Chest computed tomography scan demonstrated moderate consolidation in the right lung with pleural effusion (Figure 2). Brain computed tomography showed moderate diffuse brain atrophy without meningeal enhancement (Figure 3).
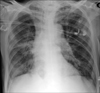
Empirical antibiotics with piperacillin-tazobactam and ciprofloxacin were administered. Because the patient's irritability was thought to be due to delirum of alcohol withdrawal, spinal tap was not performed. On the 3rd day of admission, blood cultures showed Gram-positive cocci in pairs and chains, which suggested streptococcus species. We used disc diffusion method while not utilizing automated system for antimicobial susceptiblity testing because brush dilution method was not available in our laboratory. The isolate was sensitive to penicillin, erythromycin and vancomycin. Empyema was drained through a percutaneous catheter. On hospital day 4, he had a grand mal seizure which was treated by anti-epileptic drugs. On hospital day 7, the isolated organism was identified as S. suis. Pleural fluid also grew the same organisms. A week later, the patient showed improvement with his fever and pneumonia. However, his mental status did not improve, the patient's Glasgow coma score was only was only 4 to 5 and electroencephalographic findings showed moderate cerebral dysfunction. Brain magnetic resonance imaging scans was similar with initial brain computed tomographic findings showing diffuse brain atrophy but many white blood cells were seen in cerebrospinal fluid from the patient. Cereobrospinal fluid (CSF) culture grew no organism. Those results suggested that he had meningitis and explained the reason why he did not regained consciousness. On hospital day 12, the patient acquired nosocomial pneumonia which required intubation and mechanical ventilation. On hospital day 14, multi-drug resistant Acinetobacter baumannii grew in the sputum of the patient and we switched antibiotics to intravenous colistin. On hospital day 30, pneumonia was improved so much (Figure 4) and the weaning from mechanical ventilation was done successfully. He was discharged without regaining consciousness.
S. suis, an α-hemolytic Gram-positive cocci with 35 serotypes, is an opportunistic swine pathogen, usually colonizes the palatine tonsils of both clinically ill and healthy pigs. Human infection seems to be caused by direct contact with carrier pigs, sick pigs, or raw pork contaminated with S. suis via skin wound or mucosa of the mouth and nasal cavity5. Sporadic cases of human infection have been reported in Southeast Asia and Europe, and the first human infection was reported in Denmark in 1968. Two large outbreaks of human infection were reported in the Jiangsu province (25 cases, 14 deaths) in 1998~1999, and the Sichuan province (204 cases, 38 deaths) of China in 2005, alarming healthcare providers to consider S. suis as an emerging human pathogen6. Almost all the human patients had a history of direct contact with infected pigs or pork. Person to person transmission is unlikely to occur. The incubation period of S. suis infection in the Chinese outbreak ranged from 3 hours to 14 days (median, 2.2 days)5 and most cases were reported during the warmer months, from April through October in northern Vietnam.
Human infections with S. suis manifest as meningitis (72.5%), septicemia and septic shock (24.2%), arthritis (1.1%), endocarditis (1.1%), pneumonia (0.8%) and peritonitis (0.3%)1. Meningitis is the most common clinical manifestation, often accompanied by permanent sensory neural hearing loss. Mortality is very high in septic shock patients.
Diagonosis of S. suis infection can be done by Gram stain and CSF cultures, blood or joint fluid but is commonly misidentified as Streptococcus species, viridans streptococi, Enterococcus faecalis or even S. pneumoniae 7. Therefore if α-hemolytic streptococci are cultured from patients with meningitis and sepsis, correct identification should be performed, especially in areas with high prevalence of S. suis diseases. Molecular techniques including polymerase chain reaction can be useful for diagnosis of S. suis infection even after treatment of antibiotics because S. suis DNA could still be detected. Treatment of S. suis infection is the same as those for pneumococcal meningitis because S. suis is sensitive to many antibiotics such as penicillin, ceftriaxone and vancomycin.
Very recently, two cases with S. suis infection were reported in Korea3,4. The first female case had septic arthritis and bacteremia in Chungcheong Province where many pig farms were present. The second male case was a pig carcass handler presenting meningitis with hearing loss. Both of the isolates from the patients were susceptible to penicillin, cefotaxime, ceftriaxone and vancomycin. They were cured with treatment.
This is the first case of S. suis infection presenting pneumonia with empyema in addition to septicemia and meningitis. The patient was a sailor, boarding at the South Korean ports of Yeosu on September, still hot and humid season. We could not find out whether he had exposure to contaminated pork or not. The isolate was susceptible to penicillin, erythromycin and vancomycin and the patient has improved after proper treatment. It was unfortunate obtaining the result of minimal inhibitory concentration was not done; however, the disc diffusion method we used was managed by National Committee for Clinical Laboratory Standards (NCCLS)'s quality control system, which results are reliable. Usually S. suis infection of meningitis is associated with sensoryneural hearing loss, but this case resulted neurologic sequale of severe cognitive dysfunction.
Although many cases of S. suis infection have occurred in Asia, only three cases have been reported in South Korea. This might be just the beginning and preventive measures are required by the avoidance of direct contact with contaminated pigs, wearing of gloves and adequate cooking. Clinicians and microbiologists should be aware of S. suis infection, and therefore making the correct diagnosis and proper treatment in endemic areas.
Figures and Tables
Figure 1
The initial chest X-ray shows extensive pneumonic consolidation on right upper and lower lung field.
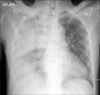
Figure 2
The chest computed tomography scan demonstrated moderate consolidation in right lung with pleural effusion.
References
1. Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007. 7:201–209.
2. Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988. 10:131–137.
3. Kim H, Lee SH, Moon HW, Kim JY, Lee SH, Hur M, et al. Streptococcus suis causes septic arthritis and bacteremia: phenotypic characterization and molecular confirmation. Korean J Lab Med. 2011. 31:115–117.
4. Huh HJ, Park KJ, Jang JH, Lee M, Lee JH, Ahn YH, et al. Streptococcus suis meningitis with bilateral sensorineural hearing loss. Korean J Lab Med. 2011. 31:205–211.
5. François B, Gissot V, Ploy MC, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol. 1998. 36:2395.
6. Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006. 12:914–920.
7. Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009. 48:617–625.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download