Abstract
Background
Adequate assessment and control of sedation play crucial roles in the proper performance of mechanical ventilation.
Methods
A total of 30 patients with various pulmonary diseases were prospectively enrolled. The study population was randomized into two groups. The sedation assessment group (SAG) received active protocol-based control of sedation, and in the empiric control group (ECG), the sedation levels were empirically adjusted. Subsequently, daily interruption of sedation (DIS) was conducted in the SAG.
Results
In the SAG, the dose of midazolam was significantly reduced by control of sedation (day 1, 1.3±0.5 µg/kg/min; day 2, 0.9±0.4 µg/kg/min; p<0.01), and was significantly lower than the ECG on day 2 (p<0.01). Likewise, on day 2, sedation levels were significantly lower in the SAG than in the ECG. Significant relationship was found between Ramsay sedation scale and Richmond agitation-sedation scale (RASS; rs=-0.57), Ramsay Sedation Scale and Bispectral Index (BIS; rs=0.77), and RASS and BIS (rs=-0.79). In 10 patients, who didn't require re-sedation after DIS, BIS showed the earliest and most significant changes among the sedation scales. Ventilatory parameters showed significant but less prominent changes, and hemodynamic parameters didn't show significant changes. No seriously adverse events ensued after the implementation of DIS.
Adequate use of sedatives is crucial in maintaining mechanical ventilation1. However, over-sedation deteriorates hemodynamic safety and oxygenation of tissues, and delays the recovery of consciousness and weaning of mechanical ventilation2. Over-sedation prevails in the majority of patients receiving mechanical ventilation. Adequate sedation is reported to be achieved merely in 20~30% of the cases3,4.
Objective indicators are essential in order to attain adequate sedation level thus, various measures are utilized. Among those, Ramsay sedation scale5 and Richmond agitation-sedation scale (RASS)6,7 are most widely used methods. The reliability and significance of these scales are verified by many researchers. When such assessment methods are applied in the process, adequate mechanical ventilation could be maintained and subsequent weaning process could be expedited. However, the sedation scale is utilized only in 30~40% of actual practices in intensive care units (ICUs)4. Likewise, utilization of the sedation scale seems to be not very active in Korea. Bispectral index (BIS) has been developed to regulate the adequate level of anesthesia by assessing electroencephalography, and its validity has been reported for objective sedation assessment during mechanical ventilation8,9. However, further studies are essential for general clinical application.
During mechanical ventilation, there are different methods of decreasing sedation level after patient conditions have been stabilized. One of the methods is gradually decreasing sedatives at a certain ratio every day. Another method is completely suspending sedation every morning (daily interruption of sedation [DIS])10-12 and re-assessing the necessity of sedatives by observing patients' conditions. Among these methods, DIS is most favorable in swift sedatives reduction. For DIS, there had been concerns on psychiatric issues, myocardial ischemia and withdrawal symptoms due to a sudden suspension of sedation. However, DIS is increasingly applied as its safety has been verified by many studies13,14.
This study aimed to implement sedation assessment and regulation during mechanical ventilation, and assess the following details. First, the study examined the degree of sedative reduction and consequently adjusted sedative levels when sedation index was actively assessed and reflected according to the protocol during the initial period of mechanical ventilation. Second, the study evaluated the aspects of changes and safety of respiratory, hemodynamic, and sedation parameters after implementing DIS during sedatives reduction.
Study population comprised patients receiving mechanical ventilation while administered with sedatives due to acute respiratory failure among 20-year-old or above inpatients in intensive care unit of Ajou University Hospital from January to June 2011. Study subjects excluded patients who are pregnant, already administered with sedatives from external hospital, revived from cardiac arrest, and suffering from brain lesions and decreased consciousness.
Patients were recorded with their demographical and clinical characteristics, causal diseases of acute respiratory failure, indices of mechanical ventilation and hemodynamic parameters. To evaluate the severity of patients, they were assessed with Acute Physiology and Chronic Health Evaluation (APACHE) II scores within the first 24 hours of hospitalization.
Subjects were divided into two groups by applying prospective, randomized, non-blind, and comparative study. Sedation regulation was conducted to sedation assessment group (SAG) according to the protocol established in advance by researchers. On the other hand, only ventilation index, hemodynamic indicators, and sedative dosage were recorded in empiric control group (ECG) in which sedatives were regulated according to subjective decision of attending physician (Figure 1).
For mechanical ventilation, assist control mode ventilation was applied and tidal volume was set with 6~8 mL/kg ideal body weight and 15~25/min of respiratory rate according to underlying diseases. The physician set peak inspiratory flow, flow waveform, I:E ratio, and positive end-expiratory pressure levels were empirically adjusted considering underlying diseases and degree of oxygenation. Ventilatory settings were continually controlled so that plateau airway pressure would not surpass 30 cm H2O. Ventilatory parameters were obtained by averaging eight times of consecutive breaths in stabilized state. Sedatives and analgesics were administered to all patients and muscle relaxants were permitted to use, if necessary, over a short period of time. Assessment of sedation was conducted at least six hours after administering muscle relaxants.
BIS of all subjects was assessed by attaching the sensors in frontal and temporal regions of patients. Sedatives were regulated by adjusting the midazolam drip and the infusion was commenced from 0.15 µg/kg/min. The midazolam doses were ranged to be between 0.07~0.5 mg/kg/hr. Pain assessments score was set to be below 5 using behavioral pain scale15.
The physician was authorized to make all decisions regarding treatment of all patients except for sedative administration. Assessment of all indicators was independently measured and recorded by two researchers. Different opinions between researchers were determined through discussion. This research had gained approval from Institutional Review Board and written consent from patients or guardians.
Sedation and other parameters were assessed on the first day when the administered dose of sedatives was thought to be fixed and ventilation and hemodynamic parameters of patients were determined to be stabilized after mechanical ventilation. Sedative level in SAG was adjusted according to the stated protocol in Figure 2. The sedative level was targeted to be 3 or 4 points of the Ramsay sedation scale5 and -2 or -3 of the RASS scores points6,7 as the moderate sedation level recommended in various protocols. BIS was only assessed and recorded, but not used for sedation control. Sedation regulation was initiated on the first day and sedation scales were assessed and recorded on the following day as stated in the protocol.
DIS was performed in SAG on the third day. Sedative administration was ceased at 10 AM on the third day in case where patients' clinical findings, hemodynamic parameters and mechanical ventilation index, and others maintained stable conditions. When patients showed changes in vital signs exhibiting agitation or demanding re-sedation due to recovery of consciousness after DIS, sedatives were re-administered in half of previous administered doses. The dosage was adjusted according to the conditions of patients. Agitation was defined as one or more points in the RASS scores. Recovery of consciousness was defined as when 1) patients open their eyes upon verbal command, 2) patients' eyes follow researchers, 3) patients stick out their tongues upon direction, and 4) patients clench their fists upon direction16. Changes in vital signs requiring re-sedation were defined as when 1) respiratory rate is 35/min or above and this rate lasts for more than five minutes, 2) oxygen saturation falls below 90%, 3) heart rate is 140/min or above or it either increases or decreases 20% from original heart rate, and 4) systolic blood pressure is 180 mm Hg or above or 90 mm Hg or below. The RASS scores, Ramsay sedation scale and BIS were assessed, and ventilation and hemodynamic parameters were recorded 1, 2, 3, 6, 12, and 24 hours after initiating DIS.
SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used in statistical analysis. Continuous variables were presented in mean and standard deviation. Mann-Whitney U test was used for comparing the differences between two groups. Categorical variables were analyzed by applying chi-square test. Spearman correlation analysis was implemented for verify the correlations among different variables. Friedman test was conducted to examine the changes within each group over time. The results were determined to be statistically significant if the p-value was less than 0.05.
Demographic and clinical characteristics of the studied subjects were described in Table 1 concerning 15 patients in each of SAG and ECG. No significant difference was found in terms of age, gender, clinical progress, APACHE II score, causal diseases, and mechanical ventilation parameters between two groups.
The infused dose of midazolam drip on the first day was 1.3±0.5 µg/kg/min and 1.4±0.5 µg/kg/min in SAG and ECG, respectively, showing no significant difference. The infused dose of midazolam drip of SAG had significantly decreased to 0.9±0.4 µg/kg/min on the second day (p<0.01) and the dose was significantly lower than that of ECG (1.3±0.5 µg/kg/min) (p<0.01) (Figure 3).
No significant difference was found in the comparison of RASS scores (-3.5±2.5 vs. -4.0±1.1, p>0.05), Ramsay sedation scales (4.7±0.9 vs. 5.0±1.2, p>0.05) and BIS scores (49.7±12.7 vs. 50.3±13.5, p>0.05) between SAG and ECG on the first day. However, a significant difference was shown in RASS scores (-3.4±0.6 vs. -4.0±1.0, p<0.05), Ramsay sedation scales (3.7±0.7 vs. 4.8±1.1, p<0.05) and BIS scores (61.0±9.8 vs. 50.0±11.7, p<0.05) between both groups on the second day.
According to correlation analysis results among sedation assessment scales in both groups, a significant correlation was found among Ramsay sedation scales, RASS scores, and BIS scores (Figure 4).
On the third day of implementing DIS in SAG, five patients were required re-administration of sedatives within six hours of follow-up period. Seven patients maintained stable conditions after recovery of consciousness and sedatives were not re-administered within 24 hours, and remaining three patients didn't require re-sedation due to delayed recovery of consciousness. To assess the changes after DIS, sedation index, and ventilation and hemodynamic indicators were recorded over time in 10 patients who were not re-administered with sedatives.
Ramsay sedation scale was 4.2±0.8 when DIS was initiated. A significant decrease to 3.8±0.8 was shown after three hours (p<0.05), and continuous significant changes were exhibited within the first 24 hours (p<0.01). The RASS scores were -3.8±0.9 and -3.4±0.8 at the time of initiation and six hours later, respectively (p=0.08), and continuous significant changes were exhibited for the first 24 hours (p<0.01). BIS was 49.4±14.3 when DIS was initiated, and it increased to 54.2±12.1 two hours later. Afterwards, BIS score was 61.1±13.4 three hours later and it had continued to increase significantly (p<0.01) (Figure 5).
With regard to changes in mechanical ventilation parameters after DIS, peak inspiratory pressure significantly increased from 29.5±2.1 cm H2O at time of initiation to 34.9±5.0 cm H2O after 24 hours (p<0.05). Spontaneous respiratory rates were 3.8±2.9/min, 5.5±2.7/min (p<0.05), and 9.0±2.7/min (p<0.01) at the time of initiating DIS, 12 hours later, and 24 hours later, respectively (Figure 6).
No significant changes were shown in peak inspiratory flow rate. Moreover, no significant changes were exhibited in tidal volume and minute ventilation due to extensive variation depending on breathing condition of patient. No significant changes were found in hemodynamic parameters including mean blood pressure and heart rates after DIS (Figure 7).
Among five patients who were re-sedated after DIS, re-sedation was implemented within 4 hours to two patients, 5 hours to a patient, and 6 hours to two patients. The changes in hemodynamic and ventilator parameters were examined until integrated analysis was possible in the group. When the values before DIS and three hours later were compared, change tendency was shown in Ramsay sedation scales (4.2±0.8 vs. 3.2±0.4, p=0.064) and RASS scores (-4.0±0.7 vs. -3.0±0.7, p=0.066). In addition, the BIS value had significantly increased from 47.4±6.4 to 58.8±11.4 (p<0.01). Spontaneous respiratory rates significantly increased from 3.2±1.4/min before DIS to 8.0±1.9/min three hours after DIS (p<0.01). Among five patients who were re-sedated, the infusion dose of midazolam drip 12 hours after DIS significantly decreased to 0.7±0.2 µg/kg/min from 1.0±0.3 µg/kg/min before DIS (p<0.05). Moreover, the dose was significantly lower than the infused midazolam dose of the third day in empirical control group (p<0.01).
No cardiovascular-related adverse effects such as myocardial ischemia were detected in DIS implemented patients. Psychiatric side effects in 10 patients who had recovered after separation from mechanical ventilation were now shown such as withdrawal symptoms of sedatives, depression, anxiety, and post-traumatic stress disorders.
As it has long been known3,4, the study has found out that there is a tendency of over-sedation in the initial stage of mechanical ventilation. Also, active assessment and control of sedation was revealed to be insufficient. Sedatives were significantly reduced through assessment and regulation of sedation. When short-term DIS was applied in limited patient group, the possibility was suggested concerning efficient and safe sedative reduction method.
When sedation control was empirically performed during mechanical ventilation, over-sedation was observed in more than half of study population. Moreover, sedation level was reported to be more deepened particularly in case of continuous intravenous infusion method during administration2,4. Continuous intravenous infusion had been conducted in this study, subsequently over-sedation was exhibited according to the assessment results of sedation scale. In previous report, active sedation regulation based on protocol was shown to be efficient in achieving appropriate sedation. Accordingly, the previous study was able to reduce 20~30% of administered volume of sedatives17,18. Significant changes in sedation scales associated with reduction in sedatives were generated by sedation regulation implemented in this study. About 30% of reduction was induced in sedative doses compare to empirical control group.
Ramsay sedation scale, RASS scores, and BIS were used in this study as the sedation scales which have been widely used in treating intensive care patients. The BIS is an objective indicator in assessing brain arousal and it is used in sedation assessment of mechanical ventilation as well as general anesthesia. BIS is reported to be correlated to other sedation indicators19,20. This study was able to verify significant correlation among sedation indicators and BIS exhibited significant results as well. One of noticeable results was that BIS showed the most sensitive change among other changes in various ventilation and sedation indicators after DIS. This finding is plausible in that the electroenphalography was reflected in BIS monitoring. Although such sensitive changes are thought to be used as significant information in patient assessment, the basis of reproducibility and objectivity is still insufficient if sedation assessment could be solely made with BIS. Moreover, it is still controversial if BIS could be generally utilized in the actual treatment of patients in ICU. Errors could be generated during analysis process due to excessive scalp muscle movement in mildly sedated patients19. It is still uncertain if BIS accurately reflects sedative state in patients using muscle relaxants20,21. In addition, maintaining BIS sensor is difficult due to edema, perspiration, movement of patients, and various procedures.
In spite of well-known clinical efficacy of sedation regulation, studies related to this subject are insufficient and clinical application tends to be inactive. Active sedation assessment is crucial for advancement and qualitative enhancement in treatment of intensive care patients in the future. Thus, attentive and proactive attitude is essential.
Among sedative reducing methods, DIS is increasingly utilized since its benefits, such as reduced mechanical ventilation and hospitalization terms, are recognized. As shown in this study, DIS was fairly tolerated, and 2/3 of DIS performed patients maintained stable ventilation without re-sedation. Swift weaning of sedatives was facilitated even in re-sedation performed patients as well by reducing the dose of sedatives10-12,21.
In case of a sudden suspension of sedatives, psychiatric side effects could be generated such as depression, anxiety, and post-traumatic stress disorders. Although there had been concerns on cardiovascular side effects such as myocardial ischemia due to sudden hemodynamic changes induced by arousal and agitation, such concerns happen very rarely in reality according to the reports of previous studies up to date. This study identified that no cardiovascular complications or serious psychiatric problems were generated in DIS-performed patients during a short follow-up period.
This study had the following limitations. First, the study was not able to conduct detailed assessment according to underlying diseases, ventilatory impairment patterns, and severity of the disease due to limited number of subjects. The study was not able to analyze possible differences in sedative demand varying depending on underlying diseases, organ dysfunction syndrome, severity and others. Second, the possibility of intervened biases or intentions was not excluded since blind test could not be applied due to the nature of this study. Third, active and efficient sedation regulation was insufficient even in SAG as shown in sedation scales. Although the researchers implemented the sedation based upon the protocol, the outcome is thought to be attributable to passive attitude due to concerns on unfamiliarity and safety regarding sedation regulation. Hence, this study would be inadequate to be comprehended as a study that has realized sufficient sedation regulation. It is thought to be reasonable to confine the definition of this study at the extent of preliminary study. Finally, the study population was insufficient to investigate on the influence of active sedation regulation on mechanical ventilation weaning, days of ICU admission, days of hospitalization, and ultimate survival. Studies including more subjects are required to further address these issues.
In conclusion, this study was able to identify that more active sedation assessment and regulation are crucial in mechanical ventilation. Efficient operation of mechanical ventilation is anticipated when more active sedation regulation is implemented using methods such as DIS, in the weaning of sedatives as well.
Figures and Tables
Figure 2
Sedation control protocol in the sedation assessment group. RASS: Richmond agitation-sedation scale.
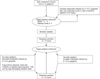
Figure 3
Comparison of intravenous midazolam doses on day 1 and 2 between the sedation assessment group and empiric control group. Data are expressed as the mean±SEM. *p<0.01 compared to both sedation assessment group on day 1 and empiric control group on day 2.

Figure 4
Correlations among sedation scales in all patients. Spearman's correlation coefficients were computed between Ramsay sedation scale and Richmond agitation-sedation scale (RASS; A), Ramsay sedation scale and Bispectral index (BIS; B), and RASS and BIS (C).

Figure 5
Changes in sedation scales after the implementation of daily interruption of sedation (DIS) in the sedation assessment group. (A) Ramsay sedation scale, (B) RASS, (C) BIS score. Data are expressed as the mean±SEM. *p<0.05 and †p<0.01 according to Friedman test. RASS: Richmond agitation-sedation scale; BIS: Bispectral index.

Figure 6
Changes in ventilator parameters after the implementation of daily interruption of sedation (DIS) in the sedation assessment group. (A) Peak inspiratory pressure, (B) Respiratory rates. Data are expressed as the mean±SEM. *p<0.05 and †p<0.01 according to Friedman test.

Figure 7
Changes in hemodynamic parameters after the implementation of daily interruption of sedation (DIS) in the sedation assessment group. (A) Mean blood pressure, (B) Heart rates. Data are expressed as the mean±SEM.

Table 1
Study population characteristics

Data are presented as mean±SD or number.
NS: not significant; APACHE: Acute Physiology and Chronic Health Evaluation; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; ALI: acute lung injury; VTe: expiratory tidal volume; IBW: ideal body weight; PEEP: positive end-expiratory pressure.
References
1. Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care. 2002. 30:119–141.
2. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998. 114:541–548.
3. Kaplan LJ, Bailey H. Bispectral index (BIS) monitoring of ICU patients on continuous infusion of sedatives and paralytics reduces sedative drug utilization and cost. Crit Care. 2000. 4:Suppl 1. P190.
4. Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007. 106:687–695.
5. Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974. 2:656–659.
6. Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003. 289:2983–2991.
7. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002. 166:1338–1344.
8. De Deyne C, Struys M, Decruyenaere J, Creupelandt J, Hoste E, Colardyn F. Use of continuous bispectral EEG monitoring to assess depth of sedation in ICU patients. Intensive Care Med. 1998. 24:1294–1298.
9. Simmons LE, Riker RR, Prato BS, Fraser GL. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit Care Med. 1999. 27:1499–1504.
10. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000. 342:1471–1477.
11. Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004. 32:1272–1276.
12. Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006. 34:1326–1332.
13. Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003. 168:1457–1461.
14. Kress JP, Vinayak AG, Levitt J, Schweickert WD, Gehlbach BK, Zimmerman F, et al. Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med. 2007. 35:365–371.
15. Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001. 29:2258–2263.
16. Kress JP, O'Connor MF, Pohlman AS, Olson D, Lavoie A, Toledano A, et al. Sedation of critically ill patients during mechanical ventilation: a comparison of propofol and midazolam. Am J Respir Crit Care Med. 1996. 153:1012–1018.
17. Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008. 36:1444–1450.
18. Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008. 36:427–433.
19. Mondello E, Siliotti R, Noto G, Cuzzocrea E, Scollo G, Trimarchi G, et al. Bispectral Index in ICU: correlation with Ramsay Score on assessment of sedation level. J Clin Monit Comput. 2002. 17:271–277.
20. Karamchandani K, Rewari V, Trikha A, Batra RK. Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. J Anesth. 2010. 24:394–398.
21. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008. 371:126–134.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download