Abstract
Background
The aim of this study was to investigate therapeutic outcomes and assess factors associated with therapeutic outcomes in hematologic patients with invasive pulmonary aspergillosis (IPA).
Methods
We analyzed all consecutive cases of IPA in adults with hematologic diseases from January 2008 to January 2009 at a Catholic Hematopoietic Stem Cell Transplantation (HSCT) Center in Seoul, Korea.
Results
A total of 54 patients were identified. Underlying diseases were acute myelogenous leukemia (n=25), acute lymphoblastic leukemia (n=10), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=3), multiple myeloma (n=3), severe aplastic anemia (n=2) and other hematologic diseases (n=4). Twenty six patients (48.2%) were assessed as having a favorable response, of which 16 patients (29.6%) showed complete response. Overall 12-week mortality and IPA attributable mortality were 38.9% (n=21) and 33.3% (n=18), respectively. In multivariate analysis, uncontrolled underlying disease (odds ratio [OR], 7.31; 95% confidence interval [CI], 1.49~35.94; p=0.014) was associated with an unfavorable response, and for 12-week mortality, uncontrolled underlying disease (OR, 11.79; 95% CI, 1.49~93.46; p=0.020) and hypoalbuminemia (OR, 9.89; 95% CI, 1.42~68.99; p=0.021) were significantly poor prognostic factors.
Invasive pulmonary aspergillosis (IPA) is the most common form of invasive aspergillosis (IA) and a major lower respiratory infection in patients with hematologic diseases. The reported mortality of IPA that developed during chemotherapy-induced neutropenia is about 60% and could exceed 90% in the setting of hematopoietic stem cell transplantation (HSCT)1,2. Over the past decade, detection of circulating galactomannan (GM) contributed to the early diagnosis of IA and new antifungal agent have improved patients' outcome3,4. However, mortality rate is still high in hematologic diseases2,5,6.
Factors affecting this poor prognosis in patients with IA have been investigated in previous studies and most of them assessed prognostic factors for survival2,5,7. Therapeutic outcome can be assessed by response to therapy as well as mortality rate5,8. Generally, allogeneic HSCT recipients, patients not in remission of their malignancy and individuals with an uncontrolled disseminated disease are shown to have the worst outcome if they have to be treated for IA.
Voriconazole (VCZ) is now considered to be the first line treatment for IA and clearly improved the clinical outcome in patients with IA. However, in Korea, many antifungal drugs including VCZ have limited use for the primary antifungal treatment due to restrictions imposed by the national health insurance system. VCZ can be covered by national health insurance only in case of clinical failure or adverse drug events by primary antifungal agents.
Here, we reviewed all consecutive cases of IPA in adults with hematologic diseases for 13 months and investigated therapeutic outcome and prognostic factors for response to therapy and mortality rate during clinical practice restricted by Korean National Health Insurance System.
We identified all cases of IPA with hematologic diseases in Catholic HSCT center from January 2008 to January 2009 and reviewed their medical records retrospectively by 2 infectious disease specialists. Patients with IPA who were enrolled in clinical trials for new antifungal agents were excluded. The following data were collected from all cases; demographic information (e.g., age and sex), underlying disease, microbiological data, radiologic findings, site of infection, antifungal agent, cytopenia, presence of graft versus host disease (GVHD), and other important clinical parameters at the time of infection. Leukocyte and differential cell counts, serum glucose and albumin level, C-reactive protein (CRP) level and creatinine clearance level were assessed using information available to the clinicians within 4 days after initiation of antifungal therapy. Chest radiography, GM serum detection by Platelia Aspergillus assay (Bio-Rad Laboratories, Marenes-la-Couquette, France) and blood chemistry were performed at least twice weekly and other examinations as clinically indicated. This study was approved by the institutional review board at St. Mary's Hospital with a waiver of informed consent (approval No. SC10RISI0011).
Proven or probable IPA was defined according to the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) definition criteria with the exception of the GM antigen test9. On the basis of previous publications10,11, we accepted as positive a single value >0.5. Patients with IA without lung involvement were excluded. When a patient developed 2 episodes of IPA, only the first episode was analyzed. Neutropenia, lymphopenia and monocytopenia were defined as a cell count less than 500, 300, and 100 per cubic millimeter, respectively12. Primary antifungal failure was assumed when the patients showed clinical failure after at least 7-days of treatment with the first-line agent or changed to another antifungal agent due to adverse drug events caused by primary agents. Patients who had experienced primary antifungal failure received salvage antifungal treatment.
Complete response was defined by the resolution of all clinical signs and symptoms attributable to IA and complete or nearly complete resolution of radiological findings. Partial response was defined by major clinical improvement and greater than 50% improvement of radiologic findings. Stable response was defined by the absence of change from baseline or an improvement of less than 50%. Failure of therapy was defined by worsening disease or death. Complete or partial response were classified as favorable response and stable response or failure were regarded as unfavorable response8. All the radiological images were analyzed by radiologists and reviewed for evaluation of response.
Overall survival and disease specific survival rates were evaluated at 12 weeks from the date of antifungal treatment active against Aspergillus species. If the cause of death was uncertain, death as a result of IPA (attributable mortality) was assumed. Overall mortality during IPA was death from any cause within 12 weeks of the diagnosis of IPA. Patients were followed up for at least 12 weeks or till time of death or follow-up loss.
Statistical significance was assessed via the χ2 test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U-test for continuous variables. We evaluated risk factors affecting unfavorable response or mortality. Variables with p-values of <0.10 in univariate analysis were entered into multivariate logistic regression analysis. Twelve-week survival was estimated by using Kaplan-Meier method and curves were compared with use of the log-rank test. p-values of less than 0.05 were considered to be statistically significant.
A total of 59 proven or probable IPA cases were identified during the study period. Five cases were excluded because these patients were enrolled into a clinical trial. Baseline dermographic and clinical characteristics of the final 54 cases are shown in Table 1. Mean age was 44±15 years (range, 21~75 years), and thirty four patients were men (63.0%). Underlying diseases were acute myelogenous leukemia (n=25, 46.3%), acute lymphoblastic leukemia (n=10, 18.5%), myelodysplastic syndrome (n=7, 12.9%), chronic myelogenous leukemia (n=3, 5.6%), multiple myeloma (n=3, 5.6%), severe aplastic anemia (n=2, 3.7%), and other hematologic diseases (n=4, 7.4%). Underlying diseases were not controlled in 18 patients (33.3%). The number of proven and probable IPA was 3 and 51, respectively. Thirty eight patients were in neutropenic state at enrollment. Among 14 allogeneic HSCT patients, 7 had GVHD. Most patients were diagnosed as IPA by GM antigen positivity rather than direct mycologic findings. Sputum culture was positive for A. fumigatus in 4 cases and A. flavus in 1 case. Four patients had a previous history of IA and concurrent cerebral (n=1), tracheobronchial (n=2), and sinonasal aspergillosis (n=1) were found in another 4 patients.
All 54 patients received antifungal therapy active against Aspergillus sp. Primary antifungal agents were amphotericin B deoxycholate (AMB, 1 mg/kg/day) in 87.0% (n=47) of patients, intravenous itraconazole (ITZ) in 9.3% (n=5), or liposomal amphotericin B (LAMB) in 3.7% (n=2). Duration of primary antifungal therapy was 10.3±6.0 days (range, 3~30 days). Reasons for change to another antifungal agent were lack of response (n=39, 72.2%), adverse drug events (n=7, 13.0%) and oral maintenance antifungal therapy (n=8, 14.8%). In cases of primary antifungal failure (n=46), intravenous VCZ (n=29) was the most common salvage antifungal agent followed by caspofungin (n=10), AMB (n=4), intravenous ITZ (n=2) and LAMB (n=1). None of the patients received combination antifungal treatment. Seven patients who received AMB as the first-line antifungal agent had to discontinue AMB due to renal failure (n=5), suspected drug rash (n=1), or infusion-related toxicity (n=1). One patient underwent right upper lobectomy on day 54 after antifungal therapy.
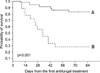
Two patients who were transferred hopelessly to other hospitals for palliative care on day 46 and 73 were presumed to be dead on day 84 and counted as deaths. All the other patients were followed up till 12 weeks after starting antifungal treatment. Diagram of enrollment and therapeutic outcome according to type of therapy are shown in Figure 1. Most patients (n=46, 85.2%) received salvage antifungal treatment. Overall, twenty six patients (48.2%) were assessed as having favorable response, of whom 16 patients (29.6%) showed complete response. Overall 30-day and 12-week mortality were 20.4% (n=11) and 38.9% (n=21), respectively. Twelve-week mortality attributable to IPA was 33.3% (n=18). IPA was not assumed to be a cause of death in 3 patients. Each patient died of septic shock by Escherichia coli, multiorgan failure with disease progression, and acute myocardial infarction. Patients with uncontrolled underlying diseases showed significantly less survival than those with remission or no evidence of relapse (survival rate, 27.8% vs. 83.2%; 95% confidence interval [CI], 13.2~58.5% vs. 71.9~96.4%, respectively; p<0.001) (Figure 2).
Results of univariate and multivariate analysis are shown in Tables 2 and 3. Uncontrolled underlying disease was a poor prognostic factor in both unfavorable response and 12-week mortality. Variables with a p-value of <0.10 in univariate analysis were entered into multivariate analysis. In multivariate analysis, uncontrolled underlying disease (odds ratio [OR], 7.31; 95% CI, 1.49~35.94; p=0.014) was associated with unfavorable response, and for 12-week mortality, uncontrolled underlying disease (OR, 11.79; 95% CI, 1.49~93.46; p=0.020) and hypoalbuminemia (OR, 9.89; 95% CI, 1.42~68.99; p=0.021) were significant poor prognostic factors.
This study investigated the therapeutic outcome and prognostic factors for clinical response and mortality in hematologic patients with IPA. The results of our study emphasized the importance of underlying disease status for outcome. Overall, fifty-nine patients were identified during the study period. Five cases were excluded from analysis because they were enrolled into a phase III double-blinded randomized clinical trial. Two cases of aplastic anemia and one case of hemophagocytic syndrome were not hematologic malignancies. But all these 3 cases had corresponding host factors predisposing to invasive fungal infections. IPA was defined according to EORTC/MSG (2002) diagnostic criteria, in which minor clinical criteria for lower respiratory tract infection include non-specific pulmonary symptoms (cough, chest pain, hemoptysis, and dyspnea), physical findings of pleural rub, any new pulmonary infiltrate or pleural effusion9. Positive chest computed tomography (CT) results suggestive of fungal infections are not always required for diagnosis of IPA. Revised criteria abandoned the distinction between "minor" and "major" clinical criteria in favor of more characteristic CT findings as lower respiratory fungal infections13. Because only 19 patients had checked chest CT scans within 4 days from starting antifungal treatment in the present study, we decided to use the 2002 definitions. To improve diagnostic accuracy, only probable and proven cases were included.
More than half of the patients (51.8%) showed unfavorable response and overall 12-week mortality was 38.9% (n=21). Eighteen (85.7%) out of 21 patients' death were attributable to IPA. Several factors could explain the poor therapeutic outcome in this study. First, most patients received AMB as first-line antifungal agent and none of the patients received VCZ as primary therapy. VCZ is now considered to be the treatment of choice for IA and clearly improved the therapeutic outcome in patients with IA4,5,14. Twelve-week overall survival was significantly higher when VCZ had been used as first-line therapy compared to other antifungal agents (p<0.016)5. However, in Korea, many antifungal agents including VCZ have limited use for the primary antifungal treatment due to restrictions imposed by the national health insurance system. VCZ can be covered by national health insurance only in case of clinical failure or adverse drug events by AMB or intravenous ITZ and due to its high cost, most patients cannot receive VCZ at their own expense. Second, the diagnosis of IPA might be delayed due to late CT scans in the course of IPA. Clearly, early diagnosis and administration of antifungal drugs are important for improving outcome of IA15,16. Forty-six patients checked chest CT scan during the course of antifungal treatment (Table 4), however, only 19 CT scans were available within 4-days of starting antifungal treatment. These delays are partially due to the attending physician's concern about infection resulting from moving the patient out of an isolation ward during neutropenic period. Among the aforementioned 19 patients, only 8 patients (42.1%) showed the halo sign which is associated with improved response and better survival16, and 12 patients (63.2%) already had bilateral lung infiltrations which are associated with increased mortality5. Greene et al.16 reported that patients presenting with a halo sign had significantly better responses to treatment (52% vs. 29%, p<0.001) and greater survival to 84 days (71% vs. 53%, p<0.01) than did patients who presented with other imaging findings. Chest X-rays are normal in many cases of early IPA and not useful for the early diagnosis of IPA2. Third, underlying hematologic diseases were not controlled in about one third of the patients. Progressive underlying malignancy has been associated with a lower overall survival and lower specific survival17-19.
Nivoix et al.5 analyzed 289 cases of IA and reported that receipt of allogeneic HSCT or solid-organ transplantation, progression of underlying malignancy, diffuse pulmonary lesions, pleural effusion, and proven or probable IA were predictors of increased overall mortality in multivariate analysis. They included possible cases of IA and patients with non-hematologic diseases. In our study, uncontrolled underlying disease was associated with both unfavorable response and 12-week mortality. Recently, Pagano et al.20 reviewed risk assessment and prognostic factors for invasive mould infection, especially, invasive aspergillus infection. Generally, allogeneic HSCT recipients, patients not in remission of their malignancy and individuals with an uncontrolled disseminated disease are shown to have the worst outcome if they have to be treated for IA. Prolonged neutropenia after chemotherapy has been reported to be associated with a poor prognosis in patients with hematological malignancies7,21. But, in our study, neutropenia was not associated with a poor therapeutic outcome, a result that may be partially due to the considerable portion of refractory patients and small number of total patients. Also, there is a possibility of "functional neutropenia" which refers to patients whose hematologic malignancy results in qualitative defects (impaired phagocytosis and killing of pathogens) of circulating neutrophils22. These patients should also be considered to be at increased risk for infection, despite a "normal" neutrophil count. Conversely, there was a report that suggested the mortality rate of IA could be higher among non-neutropenic than among neutropenic patients, including patients with hematological malignancies and solid tumors23.
Several studies proposed that serum Aspergillus GM antigen values strongly correlate with response outcome in neutropenic patients24,25. Failure of negative conversion in GM value, although statistically not significant (p=0.062) had a trend towards unfavorable response in univariate analysis in the present study. Wide variations in duration from the start of antifungal treatment to the last GM measurement (median, 38 days; range, 6~84 days) or delay in the proper antifungal treatment may influence this result in part.
CRP and albumin are acute phase proteins that increase or decrease in response to inflammation. In other clinical situations like sepsis, many studies investigated the prognostic value of CRP but most of them did not show CRP as a predictive of outcome26-28. However, acute phase reactants were not part of clinical interest as prognostic factors in fungal infection. Recently, Chai et al.29 reported that a steady decrease in the CRP profile was predictive of both clinical response and survival at week 12 in patients with IA but the CRP measurement at baseline did not predict either response or survival. In the present study, mean CRP level at baseline was lower in responders than non-responders (119 mg/L vs. 200 mg/L, p=0.003), but the 1-week decrease in CRP level was not associated with clinical response or survival. As Kontoyiannis30 indicated in the correspondence, issues about specificity and sensitivity should be resolved in future studies with a well-matched control group.
The present study has several limitations including its small, retrospective nature. First, one cannot determine the effect of different antifungal regimens because most patients received AMB as the first line therapy. Second, CT findings were excluded from analysis despite their known significance for prognosis. Third, although all the patients had hematologic diseases, some heterogenicity exits including small number of allogeneic HSCT recipients and a relatively large number of refractory patients.
In conclusion, the poor outcome of IPA in this study may be partially due to both delayed diagnosis and limited availability of VCZ as first-line therapy in Korea and our study emphasizes again the importance of underlying disease status for better prognosis in IPA. A larger study is needed to explore the influence of GM values and other clinical variables on the prognosis of IPA to allow any statistical comparison in a more selective cohort.
Figures and Tables
Figure 1
Treatment outcome and enrollment of invasive pulmonary aspergillosis according to the type of antifungal therapy used. IPA: invasive pulmonary aspergillosis; FR: favorable response; UR: unfavorable response; CR: complete response; PR: partial response; SD: stable disease.

Figure 2
Kaplan-Meier probability of overall survival after initiation of the first antifungal therapy. Solid line (A) indicates survival probability of patients (n=36) with remission or no evidence of relapse. Dotted line (B) indicates that of patients (n=18) with relapse or receiving conservative care only (median, 34 days; 95% confidence interval, 22~46 days).
Acknowledgements
We appreciate professor Jae-Wook Lee, Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea, for his careful grammatical correction of this manuscript.
References
1. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997. 175:1459–1466.
2. Subirà M, Martino R, Franquet T, Puzo C, Altés A, Sureda A, et al. Invasive pulmonary aspergillosis in patients with hematologic malignancies: survival and prognostic factors. Haematologica. 2002. 87:528–534.
3. Wheat LJ, Walsh TJ. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 2008. 27:245–251.
4. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002. 347:408–415.
5. Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008. 47:1176–1184.
6. Yoo JH, Choi SM, Lee DG, Choi JH, Shin WS, Min WS, et al. Prognostic factors influencing infection-related mortality in patients with acute leukemia in Korea. J Korean Med Sci. 2005. 20:31–35.
7. Parody R, Martino R, Sánchez F, Subirá M, Hidalgo A, Sierra J. Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplantation: single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes. Am J Hematol. 2009. 84:571–578.
8. Gallien S, Fournier S, Porcher R, Bottero J, Ribaud P, Sulahian A, et al. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department: a review of 34 cases. Infection. 2008. 36:533–538.
9. Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002. 34:7–14.
10. Herbrecht R, Letscher-Bru V, Oprea C, Lioure B, Waller J, Campos F, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J Clin Oncol. 2002. 20:1898–1906.
11. Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004. 190:641–649.
12. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007. 44:531–540.
13. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008. 46:1813–1821.
14. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008. 46:327–360.
15. von Eiff M, Roos N, Schulten R, Hesse M, Zühlsdorf M, van de Loo J. Pulmonary aspergillosis: early diagnosis improves survival. Respiration. 1995. 62:341–347.
16. Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007. 44:373–379.
17. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001. 32:358–366.
18. Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Lee JW, et al. Current trends of infectious complications following hematopoietic stem cell transplantation in a single center. J Korean Med Sci. 2006. 21:199–207.
19. Yeghen T, Kibbler CC, Prentice HG, Berger LA, Wallesby RK, McWhinney PH, et al. Management of invasive pulmonary aspergillosis in hematology patients: a review of 87 consecutive cases at a single institution. Clin Infect Dis. 2000. 31:859–868.
20. Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J Antimicrob Chemother. 2011. 66:Suppl 1. i5–i14.
21. Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010. 95:644–650.
22. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011. 52:e56–e93.
23. Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006. 43:577–584.
24. Maertens J, Buvé K, Theunissen K, Meersseman W, Verbeken E, Verhoef G, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009. 115:355–362.
25. Woods G, Miceli MH, Grazziutti ML, Zhao W, Barlogie B, Anaissie E. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer. 2007. 110:830–834.
26. Silvestre J, Póvoa P, Coelho L, Almeida E, Moreira P, Fernandes A, et al. Is C-reactive protein a good prognostic marker in septic patients? Intensive Care Med. 2009. 35:909–913.
27. Pettilä V, Pentti J, Pettilä M, Takkunen O, Jousela I. Predictive value of antithrombin III and serum C-reactive protein concentration in critically ill patients with suspected sepsis. Crit Care Med. 2002. 30:271–275.
28. Claeys R, Vinken S, Spapen H, ver Elst K, Decochez K, Huyghens L, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002. 30:757–762.
29. Chai LA, Netea MG, Teerenstra S, Earnest A, Vonk AG, Schlamm HT, et al. Early proinflammatory cytokines and C-reactive protein trends as predictors of outcome in invasive Aspergillosis. J Infect Dis. 2010. 202:1454–1462.
30. Kontoyiannis DP. Are serum cytokines sensitive and specific enough to prognosticate in aspergillosis? J Infect Dis. 2011. 203:1503.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download