Abstract
We report a rare case of lung disease caused by Mycobacterium terrae in a previously healthy woman. A 45-year-old woman was referred to our hospital due to a chronic cough with sputum. A computed tomography scan of the chest revealed bronchiolitis in conjuction with bronchiectasis in both lungs. Nontuberculous mycobacteria were identified and isolated from the bronchoalveolar lavage fluid collected from each lung. All isolates were identified as M. terrae by various molecular methods that characterized the rpoB and hsp65 gene sequences. Antibiotic therapy using clarithromycin, rifampin, and ethambutol improved the patient's condition and successfully resulted in sputum conversion.
Mycobacterium terrae complex is known to comprise multiple species, including M. terrae, M. nonchromogenicum, and M. trivia1. M. terrae is ubiquitous in the environment and generally considered a nonpathogenic nontuberculous mycboacteria (NTM)1. The most common presentation of M. terrae infection is tenosynovitis of the upper extremities, often following trauma2,3. To date, few cases of NTM lung disease caused by M. terrae have been reported. Here, we report a very rare case of M. terrae lung disease associated with bronchiectasis in an immunocompetent patient.
In March 2008, a 45-year-old woman was referred to our hospital due to chronic cough and sputum. The patient had been healthy until two months earlier, when cough and purulent sputum developed. She had a history of smoking (10 pack-years), but had stopped 10 years prior. The patient had pectus excavatum. Laboratory results were normal with the exception of elevated CA 19-9 (75.32 U/mL, normal range, 0~37 U/mL). Her height was 161 cm and body weight was 51 kg. A human immunodeficiency virus (HIV) antibody test was negative.
A computed tomography (CT) scan of the chest revealed bronchiectasis and bronchiolitis in both lungs (Figure 1A). The patient also had pectus excavatum in the anterior chest wall. There was no evidence of cystic fibrosis or other common causes of bronchiectasis. NTM were isolated from bronchoalveolar lavage fluid collected from both lungs and cultured in vitro.
Polymerase chain reaction (PCR) restriction fragment length polymorphism analysis (PRA) was used to identify the particular mycobacterial species isolated from the patient. DNA was extracted from pure culture colonies of mycobacteria grown on 7H10-OADC agar plate and the genes rpoB and hsp65 were amplified by PCR. PCR product was digested using the restriction enzymes MspI and HindIII as described previously4,5. The pattern of the resulting DNA fragments was then compared to those kept in a database of known mycobacterial species. PRA of the rpoB gene resulted in fragment lengths of 110, 95, 55, and 45 bp using MspI and 175, 55, and 45 bp using HaeIII (Figure 2). These DNA fragment lengths matched the known restriction fragment patterns of the reference strain of M. terrae ATCC15755 reported in the previous studies5,6. The identity of etiological agent was also confirmed by sequencing analysis, revealing 99 and 100% sequence similarity with the rpoB gene (GenBank accession no. AF057488.1) and hsp65 gene (GenBank accession no. AF547879) of M. terrae ATCC15755, respectively.
Drug susceptibility testing was performed using a broth microdilution method, as previously described for slow growing mycobacteria, such as M. kansasii, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI)7. The clinical isolate of M. terrae was susceptible to clarithromycin, ethambutol, and amikacin but resistant to ciprofloxacin and rifampin (Table 1).
The patient was treated with oral clarithromycin (1,000 mg/day), rifampin (600 mg/day), and ethambutol (800 mg/day) for 18 months. The treatment outcome was favorable; the patient's symptoms resolved completely, radiographic findings improved (Figure 1B), and negative conversion of sputum cultures was achieved and maintained after 12 months of antibiotic therapy (Figure 1B).
Despite the prevailing view that M. terrae complex isolates are nonpathogenic, these organisms are occasionally identified in clinical specimens8. For example, in our institution, M. terrae complex made up 3% of 1,548 NTM clinical isolates during the 2-year period from 2002 to 20039. Nonetheless, no patient had clinically significant disease caused by M. terrae complex infection during that time.
NTM lung disease caused by M. terrae complex is rarely reported in the literature10-12, and there is no reported case in Korea. Furthermore, previous studies have had difficulty identifying the exact species of clinical isolates, as commercial DNA probes and high performance liquid chromatography (HPLC) are not suitable methods to distinguish between different member species of the M. terrae complex. A recent study analyzing 16S rRNA and hsp65 genes demonstrated genomic heterogeneity among M. terrae complex members13. Therefore, we were able to confirm that our patient had M. terrae infection using modern molecular techniques that enabled sequencing of the rpoB and hsp65 genes.
NTM lung disease has two distinct radiographic manifestations: an upper lobe fibrocavitary form and a nodular bronchiectatic form1. The nodular bronchiectatic form of the disease occurs predominantly in non-smoking middle-aged or elderly women who have no underlying lung disease. The radiographic features of the nodular bronchiectatic form include bronchiectasis and multiple nodules, which tend to be most severe in the lingular segment of the left lung and in the right middle lobe. These features are well characterized in M. avium complex lung disease, but have also been noted in NTM lung disease caused by other NTM species, such as M. abscessus complex14-16. In addition, women with the nodular bronchiectatic form of NTM lung disease also often have certain medical conditions, including pectus excavatum, scoliosis, and thin body habitus17. Our case study presented with typical clinical and radiographic features of the nodular bronchiectatic form of NTM lung disease.
The optimal antimicrobial therapy regimen for M. terrae infection has yet to be established. Some have suggested that drug treatment regimen for M. terrae infection should include macrolide, rifampin, and ethambutol2. Our patient received these three drugs and had substantial improvement without significant sequelae. Despite the rifampin resistant that the clinical isolates exhibited in vitro, it was included in the treatment regimen for our patient. It is unclear whether the combination of rifampin and ethambutol proved an additive, or synergistic, effect2.
In conclusion, M. terrae should be considered a possible etiologic pathogen of the nodular bronchiectatic form of NTM lung disease, despite the rare occurrence of M. terrae lung disease. Additionally, macrolide-containing antibiotic therapies may be effective in the treatment of M. terrae lung disease.
Figures and Tables
Figure 1
A 45-year-old woman with bronchiectasis and nontuberculous mycobacterial lung disease caused by Mycobacterium terrae. (A) A transverse CT scan (2.5-mm-section thickness) obtained on level with the right inferior pulmonary vein at the time of presentation, before treatment, reveals bilateral bronchiectasis (arrows) in the right middle lobe and the lingular segment of the left upper lobe, as well as cellular bronchiolitis with tree-in-bud signs (black arrow heads) in both lungs. Lobular consolidation in the right middle lobe (white arrow heads) was also noted. (B) A CT scan of the same patient after 12 months of antibiotic therapy revealed a decrease in the extent of cellular bronchiolitis and lobular consolidation in both lungs. CT: computed tomography.
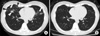
Figure 2
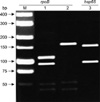
Electrophoresis of the PCR restriction enzyme PRA of the rpoB and hsp65 genes from the clinical isolate of the patient. M, size marker; Lane 1, rpoB gene PCR product after digestion by MspI Lane 2, PCR product of the rpoB gene after digestion by HaeIII Lane 3, PCR product of the hsp65 gene after digestion by HaeIII. PCR: polymerase chain reaction; PRA: polymorphism analysis.
Acknowledgements
This study was financially supported by research fund of Chungnam National University in 2011 (2011-1587).
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.
2. Smith DS, Lindholm-Levy P, Huitt GA, Heifets LB, Cook JL. Mycobacterium terrae: case reports, literature review, and in vitro antibiotic susceptibility testing. Clin Infect Dis. 2000. 30:444–453.
3. Chen HW, Lai CC, Tan CK. Arthritis caused by Mycobacterium terrae in a patient with rheumatoid arthritis. Int J Infect Dis. 2009. 13:e145–e147.
4. Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993. 31:175–178.
5. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000. 38:2966–2971.
6. Alban SM, Sella SR, Miranda RN, Mira MT, Soccol VT. PCR-restriction fragment length polymorphism analysis as a tool for Mycobacterium species identification in lepromas for lepromin production. Lepr Rev. 2009. 80:129–142.
7. Clinical and Laboratory Standards Institute (CLSI). Document no, M24-A. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. 2003. Wayne, PA: CLSI.
8. Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992. 5:1–25.
9. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–348.
10. Kuze F, Mitsuoka A, Chiba W, Shimizu Y, Ito M, Teramatsu T, et al. Chronic pulmonary infection caused by Mycobacterium terrae complex: a resected case. Am Rev Respir Dis. 1983. 128:561–565.
11. Krisher KK, Kallay MC, Nolte FS. Primary pulmonary infection caused by Mycobacterium terrae complex. Diagn Microbiol Infect Dis. 1988. 11:171–175.
12. Tonner JA, Hammond MD. Pulmonary disease caused by Mycobacterium terrae complex. South Med J. 1989. 82:1279–1282.
13. Lee CK, Gi HM, Cho Y, Kim YK, Lee KN, Song KJ, et al. The genomic heterogeneity among Mycobacterium terrae complex displayed by sequencing of 16S rRNA and hsp 65 genes. Microbiol Immunol. 2004. 48:83–90.
14. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005. 235:282–288.
15. Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009. 180:896–902.
16. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011. 183:405–410.
17. Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008. 178:1066–1074.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download