Abstract
Background
It is known that cigarette smoke (CS) causes cell death. Apoptotic cell death is involved in the pathogenesis of CS-related lung diseases. Some members of the protein kinase C (PKC) family have roles in cigarette smoke extract (CSE)-induced apoptosis. This study was conducted to investigate the role of PKC epsilon in CSE-induced apoptosis in human lung fibroblast cell line, MRC-5.
Methods
Lactate dehydrogenase release was measured using a cytotoxicity detection kit. The MTT assay was used to measure cell viability. Western immunoblot, Hoechst 33342 staining and flow cytometry were used to demonstrate the effect of PKCε. Caspase-3 and caspase-8 activities were determined using a colorimetric assay. To examine PKCε activation, Western blotting was performed using both fractions of membrane and cytosol.
Results
We showed that CSE activated PKCε by demonstrating increased expression of PKCε in the plasma membrane fraction. Pre-treatment of PKCε peptide inhibitor attenuated CSE-induced apoptotic cell death, as demonstrated by the MTT assay (13.03% of control, 85.66% of CSE-treatment, and 53.73% of PKCε peptide inhibitor-pre-treatment, respectively), Hoechst 33342 staining, and flow cytometry (85.64% of CSE-treatment, 53.73% of PKCε peptide inhibitor-pre-treatment). Pre-treatment of PKCε peptide inhibitor reduced caspase-3 expression and attenuated caspase-3, caspase-8 activity compared with CSE treatment alone.
Cigarette smoke (CS) is a major risk factor for lung diseases characterized by chronic airway inflammation and parenchymal destruction such as chronic obstructive pulmonary disease. Protease/antiprotease imbalance and oxidative stress caused by CS have been suggested mechanisms for the development of CS-related lung diseases. However, recent studies suggested that apoptosis also might be important to the pathogenesis of CS-related lung diseases and a contributing factor to the destruction of lung tissues in response to CS1. Several studies demonstrated that CS induced apoptosis and apoptogenic factors in vivo and in vitro experiments2-4. Intrinsic mitochondria-dependent apoptotic pathway was regarded as main activating pathway induced by cigarette smoke extract (CSE) in alveolar macrophages4. However, another studies reported high level of proapoptotic proteins (caspase-3, caspase-8, Bax, truncated Bid, and cytochrome c) in the lungs of CS-exposed rats5,6, which indicated that CS sensitizes lung cells toward apoptosis via both extrinsic and intrinsic pathways. The precise molecular mechanisms underlying the apoptotic pathways triggered and regulated by CS remain to be further investigated.
The protein kinase C (PKC) family consists of at least 12 broadly expressed serine/threonine kinase isoforms. Based on their regulation mechanism, PKCs are divided into three subgroups; the conventional PKCs (α, βI, βII, and γ), the novel PKCs (δ, ε, θ, µ and η), and the atypical PKCs (ζ, λ, and τ). The protein kinase C (PKC) family is responsible for transducing many cellular signaling during cell death7-9. Recently, the role of PKC family in CSE-induced apoptosis was reported10. Researchers demonstrated that CSE induced Fas receptor-mediated death inducing signaling complex (DISC) formation, which was differentially regulated by PKCα and PKCζ via the PI3K/Akt pathway10. This finding prompted us to investigate whether PKCs have isoform-specific role in CSE-induced apoptosis. Here, we investigated the role of PKCε in CSE-induced apoptosis. And we demonstrated that inhibition of PKCε attenuated CSE-induced apoptotic cell death and the extrinsic apoptotic pathway was responsible for CSE-induced apoptotic cell death in MRC-5 cell.
Kentucky 1R3F research-reference filtered cigarettes (The Tobacco Research Institute, University of Kentucky, Lexington, KY, USA) were smoked using a peristaltic pump (VWR International). Before the experiments, the filters were cut from the cigarettes. Each cigarette was smoked in 6 minutes with a 17-mm butt remaining. Four cigarettes were bubbled through 40 mL of cell growth medium, and this solution, regarded as 100% strength CSE, was adjusted to a pH of 7.45 and used within 15 minutes after preparation.
Human lung fibroblast cell line, MRC-5, was purchased from ATCC (Manassas, VA, USA). The MRC-5 cells were cultured in DMEM (Gibco-BRL, Grand Island, NT, USA) supplemented with 10% FBS at 37℃ in a humidified atmosphere containing 5% CO2. For CSE treatment, the MRC-5 cells were plated at 50~60% confluence. Cells were also pretreated with 10µM PKCε peptide inhibitor (Santa Cruz, CA, USA) for 3 hours and followed by exposure to 20% CSE.
Lactate dehydrogenase (LDH) release was measured using a cytotoxicity detection kit (TaKaRa, Shiga, Japan), according to the manufacturer's protocol. After gentle agitation, 100µL of culture medium was collected at specific time for the assay. Cell viability was quantified by using MTT (Sigma, St. Louis, MO, USA) reagent (5 mg/mL) added into each well. After 2 hours incubation at 37℃ the media were removed, and the intracellular formazan product was dissolved in 250µL of DMSO. The absorbance was measured at 540 nm using the microplate reader (Thermo scientific, Waltham, MA, USA).
Apoptosis was investigated by staining the cells with Hoechst 33342 (Sigma, St. Louis, MO, USA). MRC-5 cells were washed twice with PBS and then fixed in PBS containing 4% formaldehyde for 10 minutes at room temperature. Fixed cells were washed with PBS and stained with Hoechst 33342 for 5 minutes at room temperature. Cells were evaluated under a fluorescence microscope (OlympusIX70; Olympus Corp., Tokyo, Japan) for nuclei showing typical apoptotic features such as chromatin condensation and fragmentation. Photographs were taken at a magnification of ×400.
Surface exposure of phosphatidyl serine in apoptotic cells was quantitatively detected using the annexin V-FITC and propidium iodide (PI) apoptosis detection kit (Beckton Dickinson, San Jose, CA, USA). Briefly, cells were seeded into 6-well plates (1.0×106 cells/mL) and incubated for 24 hours. MRC-5 cells were pretreated with 10µM of PKCε peptide inhibitor for 3 hours and then exposed to 20% CSE for 12 hours. After 5 minutes of centrifuging at 5,000 rpm, annexin V-FITC and PI double-staining were performed according to manufacturer's instruction. Cell apoptosis was analysed on a FACSscan flow cytometer (Beckton Dickinson). Annexin V-FITC-positive, PI-negative cells were scored as apoptotic.
Caspase-3 and -8 activities were determined by a colorimetric assay using kits from R&D System (Wiesbaden- Nordenstadt, Germany), according to the manufacturer's protocol. Briefly, cells were lysed in the supplied lysis buffer and were incubated on ice for 10 minutes. At the end of the incubation, cell lysates were centrifuged at 10,000×g for 10 minutes at 4℃ to precipitate cellular debris. The supernatants were collected and incubated with the supplied reaction buffer containing dithiothreitol and DEVD-pNA (specific for caspase-3) or IETD-pNA (specific for caspase-8) as substrates at 37℃. The reaction was measured by absorbance at 405 nm using the microplate reader (Thermo scientific, Waltham, MA, USA). Enzyme activity was expressed as the fold increase in the proportion of apoptotic cells over that of non-treated control cells.
Cells were lysed in ahypotonic buffer (20 mM HEPES [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, protein inhibitor cocktail). The lysates were passed through a 25 gauge needle 10 times using a 1 mL syringe and leave on ice for 20 minutes. After incubation lysates were centrifuged 100,000 g for 1 hour at 4℃, obtaining supernatant as a soluble (cytosolic) fraction. The pellet was resuspended in 1% Triton X-100-containing buffer and centrifuged 10,000 g for 20 minutes at 4℃. The supernatant was recovered as a membrane fraction. The samples were resolved on 8% and 12% SDS-polyacrylamide gel, transferred to PVDF membrane (Millipore, Bedford, MA, USA) and reacted with antibodies of PKC epsilon (Santa Cruz, CA, USA), cleaved-caspase 3 (Cell Signaling, Boston, MA, USA). Immunoreactive bands were visualized using an enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK). Immunoreactivity was analyzed by densitometry using program Image J (National Institutes of Health, Bethesda, MD, USA). Protein concentration was determined using the bicinchoninic acid assay (Bio-Rad, Richmond, CA, USA).
We investigated the effect of CSE on MRC-5 cell viability using MTT and LDH release assay (Figure 1). MRC-5 cells were treated with 20% CSE for 0, 6, 12, 24, and 48 hours for the MTT assay. CSE decreased cell viability more than 12 hours exposure in a time-dependent manner, with the significant reduction in cell viability (28%) occurring at 20% CSE compared with control (Figure 1A). For LDH release assay, MRC-5 cells were exposed to 20% CSE for 0, 6, 12, 24, and 48 hours. As shown in Figure 1B, LDH release following CSE exposure was significantly increased in a time-dependent manner relative to untreated cells. Thus, subsequent observations in this study were made at 20% CSE concentration and kinetic combinations that preceded the appearance of significant LDH release or loss of viability. Caspase-3 is an executioner caspase. Its activation represents a distal event in apoptosis-signaling pathways. Hence, we investigated whether CSE exposure may activate apoptotic pathway and observed a clear expression of cleaved caspase-3 subunit p19 up to 24 hours after 6 hours of 20% CSE exposure (Figure 1C). These results indicate that CSE exposure induces MRC-5 cell death through the apoptotic pathway.
To investigate whether CSE can activate PKCε, MRC-5 cells treated with 20% CSE for 12 hours were fractionated into membrane and cytosolic fractions and both fractions were immunoblotted with antisera against PKCε, as described in Materials and Methods. After 12 hours of CSE exposure, the membrane fraction of MRC-5 exhibited increased PKCε (Figure 2A), indicating activation of PKCε by CSE exposure. Pretreatment of PKCε peptide inhibitor attenuated the activation of PKCε induced by CSE (Figure 2A). We questioned whether PKCε inhibition could protect cell death cause by CSE exposure in MRC-5 cells. To investigate the effect of PKCε inhibition on cell death, we pretreated MRC-5 cells with PKCε peptide inhibitor (1, 3, 10, and 30µM), and then exposed the pretreated-MRC-5 cells to 20% CSE for 12 hours. Surprisingly, PKCε inhibition attenuated cell death caused by CSE exposure in a dose-dependent manner (Figure 2B). High concentration (10 and 30µM) of PKCε peptide inhibitor nearly completely protected cell death induced by CSE exposure. These results suggest that PKCε could be an important molecule closely related with CSE-induced cell death.
We examined whether the protective role of PKCε inhibition in CSE-induced cell death is apoptosis-mediated effect with flow cytometry analysis. MRC-5 cells were pretreated with PKCε peptide inhibitor (10µM) for 3 hours and then exposed to 20% CSE for 12 hours (Figure 3A). The rate of apoptosis of untreated, CSE-treated, and PKCε peptide inhibitor-treated cells were 13.03%, 85.66%, and 53.73%, respectively (Figure 3A, B). Treatment of PKCε peptide inhibitor significantly decreased apoptosis compared to CSE treatment (p<0.05) (Figure 3A, B). We also performed Hoechst 33342 staining to examine morphological changes. Chromosomal condensation and nuclear fragmentation were observed in many MRC-5 cells exposed to 20% CSE for 12 hours (Figure 3C). Treatment of PKCε peptide inhibitor decreased the number of Hoechst 33342 stained nuclei compared with 20% CSE treatment. PKCε peptide inhibitor-treated MRC-5 cells showed fewer morphological changes than CSE treated cells in phase contrast microscopy (Figure 3C, right column). These results indicate that PKCε inhibition protects CSE-induced apoptotic cell death.
Previous study demonstrated that CSE induces MRC-5 cell death through the extrinsic apoptotic pathway10. Hence, we further investigated the apoptotic pathway of CSE-induced PKCε activation focusing on extrinsic pathway. Because activation of caspase-8 represents an initial event in extrinsic apoptotic signaling pathways, we assessed caspase-3 and caspase-8 activity using immunoblotting and colorimetric assay. Treatment of PKCε peptide inhibitor attenuated caspase-3 activity significantly compared with CSE treatment in MRC-5 cells (Figure 4A, B). CSE exposure for 12 hours increased caspase-3 and caspase-8 activities in MRC-5 cells. Treatment of PKCε peptide inhibitor significantly decreased the caspase-3 and caspase-8 activities compared with CSE treatment (Figure 4C, D). These results indicate that CSE-induced apoptotic cell death and PKCε inhibition-induced antiapoptotic effect in MRC-5 cells exposed to CSE are dependent on the activity of extrinsic apoptotic pathway.
The mechanism of apoptosis in CSE-induced cell death remains incompletely understood11,12. In this study, we demonstrated that PKCε activation induced by CSE exposure is involved in a critical mechanism of CSE-induced apoptotic cell death in MFC-5 cells. Translocation from cytosol to plasma membrane is a preferential mechanism involved in the activation of PKCα, ζ, ε, and η isoforms10. We showed CSE-induced PKCε activation by demonstrating increased expression of PKCε in plasma membrane fraction of the cell lysates. Treatment of PKCε peptide inhibitor decreased PKCε expression in plasma membrane fraction, which was well correlated with decreased the number of apoptotic cells assessed by Hoechst 33342 staining and flow cytometry (Figure 3). PKCε peptide inhibitor also decreased caspase-3 cleavage (Figure 2A). To our knowledge, this is the first report to demonstrate that PKCε activation is critically mediated with apoptotic cell death induced by CSE exposure in human fibroblast cells.
PKCε seems to play apoptotic or antiapoptotic roles differentially dependent on cell- or injury-specificity. PKCε played a protective role against oxidative stress-induced injury in HT22 cells13 and ischemia-reperfusion injury in neurons14. In contrast, PKCε have proapoptotic functions in cardiomyocytes15 and NIH3T3 cells16. The biological functions of PKCε of human lung fibroblast exposed to CSE are not known. In the present study, we showed that PKCε inhibition of MRC-5 cells under CSE-exposure decreased apoptosis (Figure 3A, B) and decreased the caspase-3 and caspase-8 activities significantly compared with CSE exposure only (Figure 4C). Although, in addition to inhibition of PKCε activity by pharmacologic inhibitor, transient or stable knockdown of endogenous PKCε by RNA interference or established stable clones with short hairpin RNA-mediated suppression of PKCε may help to understand the exact role of PKCε in CSE-induced apoptotic cell death, our results indicate that PKCε may have proapoptotic function in CSE-exposed MRC-5 cells. These results are consistent with previous studies demonstrating proapoptotic role of PKCε17-19.
The relative role of individual PKC isoforms in Fasmediated apoptosis and caspases activation remains unclear. Previously, a study demonstrated that CSE induces MRC-5 cell death through the extrinsic apoptotic pathway10. In this study, we further observed that extrinsic apoptotic pathway was also responsible for PKCε inhibition-induced antiapoptotic effect in MRC-5 cells exposed to CSE.
We used only one cell type (fibroblasts) to investigate the role of PKCε in apoptosis induced by CS-exposure. It is unclear whether PKCε under CS exposure may play the same roles in the other cellular components of the lung, such as epithelial cells, endothelial cells, smooth muscle cells, pneumocytes, and alveolar macrophages. Notably, Kim and colleagues have observed that CS induces DISC formation and caspase-8 activation in human bronchial epithelial cells (Beas-2B)20. This result is consistent with ours. Therefore it may be valuable to study the inhibition of PKCε may have beneficial effects on CS-related lung diseases of which CS-induced apoptotic cell death is important for pathogenesis and progression.
Taken together, this study indicates that activation of PKCε induced by CSE exposure is a critical mechanism of CSE-induced apoptotic cell death in MRC-5 cells. And PKCε seems to have proapoptotic function and exert its function through extrinsic apoptotic pathway in CSE-exposed MRC-5 cells. In the clinical view point, this study suggests that PKCε inhibition may be a therapeutic strategy in CS-related lung disease such as chronic obstructive pulmonary disease.
Figures and Tables
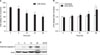 | Figure 1CSE induces MRC-5 cell death. (A) MRC-5 cells were exposed to 20% CSE at the indicated times, and cell viability was assessed using the MTT assay. (B) MRC-5 cells were exposed to 20% CSE at the indicated times, and cell cytotoxicity was assessed LDH assay. Data from each time point were compared with untreated cells at each time points. (C) MRC-5 cells were treated with 20% CSE for the indicated time periods. Activation of caspase-3 was assessed by Western blotting in total cell lysates. Data are expressed as the mean±SE. Significance was determined using t-test. *p<0.05, †p<0.005. CSE: cigarette smoke extract; LDH: lactate dehydrogenase; SE: standard error. |
 | Figure 2PKCε inhibition increases cell viability. (A) Translocation of PKCε from cytosol to the membrane fraction was analyzed in MRC-5 treated with 20% CSE for 12 hours. Expression of PKCε was detected by Western blot analysis as described. Cytosol and membrane fractions were isolated as described in Materials and Methods. All results are representative of three independent experiments. (B) Cell viability was evaluated after 12 hours incubation at the different concentration of PKCε peptide inhibitor using the MTT assay as described in Materials and Methods. Data are expressed as the mean±SE. Significance was determined using t-test. *p<0.05, †p<0.005. PKCε: protein kinase C epsilon; CSE: cigarette smoke extract; SE: standard error. |
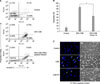 | Figure 3Flow cytometry analysis using annexin V and PI staining and Hoechst 33342 staining. (A) The apoptosis level of MRC-5 cells pretreated with PKCε peptide inhibitor, 20% CSE exposure alone, or control was compared using flow cytometry. Results are representative of three independent experiments. Annexin V (+) PI (-) cells (right lower) are considered apoptotic. (B) Histogram represents data from flow cytometry. Data are expressed as the mean±SE. Significance was determined using t-test. (C) Nuclei of MRC-5 cells were stained with Hoechst 33342 to detect apoptosis morphologically. White arrows point to the fragmented condensed nuclei. Photographs are representative results from three independent experiments. Images from phase contrast microscopy were also displayed (×200). *p<0.05. CSE: cigarette smoke extract; PI: propidium iodide; PKCε: protein kinase C epsilon; SE: standard error. |
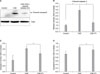 | Figure 4Inhibition of PKCε decreases caspase-3 and caspase-8 activities in MRC-5 cells after CSE exposure. (A) MRC-5 cells were pretreated with PKCε peptide inhibitor and Western blotted with cleaved caspase-3. (B) Histogram represents results from Western blotting. (C, D) The caspase-3 and caspase-8 activities were assessed by colorimetric assay. Data are expressed as the mean±SE. Significance was determined using t-test. *p<0.05. CSE: cigarette smoke extract; PKCε: protein kinase C epsilon; SE: standard error. |
Acknowledgements
This study was supported by a 2010 grant from The Korean Academy of Tuberculosis and Respiratory Diseases (to Jeong-Woong Park).
References
1. Sakao S, Tatsumi K, Hashimoto T, Igari H, Shino Y, Shirasawa H, et al. Vascular endothelial growth factor and the risk of smoking-related COPD. Chest. 2003. 124:323–327.
2. Sugano N, Ito K. Nicotine switches the form of H2O2-induced cell death from apoptosis to necrosis in U937 cells. Immunol Lett. 2000. 72:163–166.
3. Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001. 72:82–88.
4. Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L1392–L1401.
5. Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD, Wang CJ. Induction of apoptosis in the lung tissue from rats exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol Interact. 2005. 155:31–42.
6. Wu CH, Lin HH, Yan FP, Wu CH, Wang CJ. Immunohistochemical detection of apoptotic proteins, p53/Bax and JNK/FasL cascade, in the lung of rats exposed to cigarette smoke. Arch Toxicol. 2006. 80:328–336.
7. Wang X, Zhao J, Tang S, Lee S, Glazer RI, Hewlett I. c-FLIPL regulates PKC via AP-2 to inhibit Bax-mediated apoptosis induced by HIV-1 gp120 in Jurkat cells. Mol Cell Biochem. 2009. 330:23–29.
8. Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK. PKC delta signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy. 2009. 5:244–246.
9. Li X, Pabla N, Wei Q, Dong G, Messing RO, Wang CY, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010. 21:1115–1124.
10. Park JW, Kim HP, Lee SJ, Wang X, Wang Y, Ifedigbo E, et al. Protein kinase C alpha and zeta differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J Immunol. 2008. 180:4668–4678.
11. Kim H, Liu X, Kobayashi T, Conner H, Kohyama T, Wen FQ, et al. Reversible cigarette smoke extract-induced DNA damage in human lung fibroblasts. Am J Respir Cell Mol Biol. 2004. 31:483–490.
12. Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, et al. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2005. 33:121–129.
13. Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001. 21:2929–2938.
14. Reshef A, Capua ND, Sperling O, Zoref-Shani E. Ischemic tolerance conferred to cultured rat neurons by heat shock is not mediated by opening of adenosine triphosphate-sensitive potassium channels. Neurosci Lett. 2000. 287:223–226.
15. Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta-adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001. 33:1791–1803.
16. Lee YJ, Soh JW, Jeoung DI, Cho CK, Jhon GJ, Lee SJ, et al. PKC epsilon-mediated ERK1/2 activation involved in radiation-induced cell death in NIH3T3 cells. Biochim Biophys Acta. 2003. 1593:219–229.
17. Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997. 94:11233–11237.
18. Ha H, Yu MR, Choi YJ, Lee HB. Activation of protein kinase c-delta and c-epsilon by oxidative stress in early diabetic rat kidney. Am J Kidney Dis. 2001. 38:4 Suppl 1. S204–S207.
19. Jung YS, Ryu BR, Lee BK, Mook-Jung I, Kim SU, Lee SH, et al. Role for PKC-epsilon in neuronal death induced by oxidative stress. Biochem Biophys Res Commun. 2004. 320:789–794.
20. Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008. 4:887–895.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download