Abstract
Pulmonary lipiodol embolism is a rare but very fatal complication of transcatheter arterial chemoembolization (TACE), Here we present the case of an unusual complication of TACE in a 67-year-old man who presented with dyspnea, hemoptysis, and a history of a third session of TACE for hepatocellular carcinoma (HCC) that had been performed 3 days prior to presenting. On the basis of chest X-ray and computed tomography (CT) scan findings, we diagnosed pulmonary lipiodol embolism. He was conservatively treated with oxygen and haemostatic agents. The patient recovered quickly without any significant sequela and was discharged.
Pulmonary embolism is defined as the blockage of pulmonary vasculatures by a substance that has migrated from another location in the body via the bloodstream. It usually occurs because of a thromboembolism arising from the deep veins in the legs, but is rarely due to other causes such as parasites, air, fat, amniotic fluid and talc1.
Pulmonary lipiodol embolism is a very rare but fatal complication associated with transcatheter arterial chemoembolization (TACE). Here, we report a case of pulmonary lipiodol embolism in a patient who presented with mild hemoptysis and dyspnea after TACE, which was performed for hepatocellular carcinoma (HCC).
A 67-year-old man was admitted to our hospital for the evaluation and treatment of hemoptysis and dyspnea of one day duration. He was diagnosed with hepatitis B virus-associated liver cirrhosis 3 years ago, and with hepatocelluar carcinoma 5 months ago. He had undergone TACE for his hepatocellular carcinoma. The third TACE session had been performed 3 days ago, after procedure he experienced mild dyspnea, dry cough, and small amount hemoptysis old color but he was discharged.
On admission, he had dyspnea (modified ATS Class III), the total 70 mL amount of hemptysis and fifteen times blood tinged sputum until visit hospital, and his vital signs were as follows: blood pressure, 130/80 mm Hg; pulse rate, 90 beats per minute; respiratory rate, 24 breaths per minute; and body temperature, 36.6℃.
During physical examination, the patient appeared chronically ill, and chest auscultation revealed mild crackle sound in the right lower lung field; other respiratory system examination findings were nonspecific, he doesn't appear cyanotic.
Blood examination showed a hemoglobin level of 12.0 g/dL, white blood cell count of 10.7×103/mL (neutrophils 87.3%, lymphocytes 6.6% and monocytes 3.1%), and platelet count of 67×103/µL.
Biochemical profiling revealed aspartate aminotransferase (AST), alanine aminotranferase (ALT) and total bilirubin were 103 IU/L (normal range, 0~40 IU/L), 71 IU/L (normal range, 0~40 IU/L) and 1.86 mg/dL (normal range, 0~1.2 mg/dL), respectively.
Other biochemical parameters were within their normal range. The echocardiograhy and D-dimer were not examined.
At room air, arterial blood gas analysis revealed the following: pH 7.466, PaCO2 27.8 mm Hg, PaO2 58.5 mm Hg, HCO3 - , 19.8 mm Hg, SaO2 88%, PAO2-PaO2 56.75 mm Hg.
After 5 L/min oxygen supplement via nasal prong, his oxygenation was not changed, and these findings were compatible with shunt.
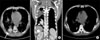
The chest X-ray film revealed new consolidation in the right lower lung fields (Figure 1A, B). Chest computed tomography (CT) scan without contrast enhancement was performed to evaluate the atypical presentation. It revealed high-density lipiodol deposition in the right lower lobe of the lung (Figure 2A).
Subsequently, oxygen were supplied and intra-venous tranexamic acid 500 mg were administered every 8 hours for symptomatic treatment, the treatment alleviated his symptoms and improved his overall condition.
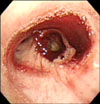
On the third hospital day, bronchoscopy was performed for the differential diagnosis of hemoptysis (Figure 3). Bronchioalveolar lavage was performed in posterobasal segmental bronchus of right lower lung, and examination of the fluid was revealed the following: red blood cell count 48,250/µL, white blood cell count 320/µL, polynuclear cell 49%, mononuclear cell 51%. These findings were compatible with pulmonary hemorrhage.
Continuous symptomatic treatment was administered, and the follow-up chest radiography revealed partial decrease in the pulmonary infiltrates (Figure 1C).
On the seventh hospital day, the patient was discharged after much symptomatic improvement.
A month later, follow-up chest CT scan revealed improved lipiodol uptake within the lung parenchyma of the right lower lobe without any respiratory sequela (Figure 2B).
HCC is the fifth most prevalent cancer in Korea2. The treatment of HCC is divided based on the different stages of cancer. Surgical resection and liver transplantation are performed in operable HCC patients. Whereas, systemic chemotherapy and TACE are performed in inoperable HCC patients. However, systemic chemotherapy is not effective for HCC. Therefore, currently TACE has become an effective treatment strategy for inoperable HCC patients.
Although TACE is relatively safe, It have relevance to rare but serious complications. For example, hepatic abscess, hepatic infarction, acute hepatic failure, spontaneous rupture of the tumor, gastrointestinal mucosal ulceration, and acute renal failure have been reported3,4.
Pulmonary lipiodol embolism is also a rare but fatal complication associated with TACE. Samejima et al.5 were the first to report pulmonary complications of TACE. However, a large prospective study on this complication has not been performed yet. Kita et al.6 reported about 23% of abnormal pulmonary perfusion scans were obtained after TACE, but reported that the development of respiratory symptoms was uncommon. The incidence of symptomatic pulmonary lipiodol embolism ranged between 0.05~1.8%7.
The followings have been considered as the risk factors of pulmonary lipiodol embolism: The dose of iodized oil injected, the presence of an arteriovenous shunt, and fistula between the inferior phrenic artery and an artery of the pulmonary circulation8.
In our case, the lipiodol material might have embolized and then circulated via the fistula between the right inferior phrenic artery branch and the right pulmonary artery (Figure 4).
Because we found that the used lipiodol dosage used was 7 mL, this dose is not usually considered to cause lipiodol embolism. Furthermore, we found that there was no arteriovenous shunt.
While a thrombi usually obstructs the main branch of an artery in the pulmonary circulation, the lipiodol emboli shows a diffuse distribution to peripheral blood vessels similar to the case in fat embolism.
Therefore, it is important to know the characteristic radiological pattern of pulmonary lipiodol embolism. Chest X-ray film shows an increased ill defined opacity and the chest CT scan reveals new multiple iodized oil-like high-density materials in the parenchyma of the lung after TACE3.
Further, the medical team should also know the manifestations of lipiodol embolism, namely, dyspnea, dry cough, and hemoptysis. In our case, the medical team ignored the symptom of mild dyspnea, which appeared after TACE, and the patient discharged at the next morning.
On the third day after discharge, the patient presented to our emergency room because of aggravated dyspnea and a fresh hemoptysis and was diagnosed with pulmonary lipiodol embolism.
The effective and targeted therapy of lipiodol embolism had not known yet. Nevertheless, immediate symptomatic care should be started including oxygen supply, high dosage methylprednisolone, heparin, ventilator care for respiratory failure9, and, careful monitoring of the patient's respiratory status by checking the oxygen saturation, vital signs, and mental status until his symptoms disappeared.
In our patient, oxygen therapy and haemostatic agent were effective and brought symptomatic relief.
In conclusion, we should be observant about this rare, but serious pulmonary complication that may occur after transcatheter arterial chemoembolization, and we should carefully monitor the patient for signs of development of pulmonary lipiodol embolism during and after procedure of transcatheter arterial chemoembolization.
Figures and Tables
Figure 1
Chest radiography. (A) Prior to TACE, the chest X-ray is normal findings. (B) On admission, the chest X-ray shows ill-defined multiple opacity in the right lower lung field. (C) On the seventh day, the chest X-ray shows a slight decrease in the opacity in the right lower lung field. TACE: transcatheter arterial chemoembolization.
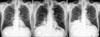
Figure 2
Chest CT. (A) Pre-contrast CT scan shows small amount of pleural effusion and consolidation with internal high density in the right lower lobe. (B) After 1 month, follow-up CT scan shows the disappearance of pleural effusion and greater reduction in the consolidation with internal high density. CT: computed tomography.
References
1. Morris TA, Fedullo PF. Mason RJ, Broaddus C, Murray JF, Nadel JA, editors. Disorders of the pulmonary circulation. Murray and Nael's textbook of respiratory medicine. 2005. 4th ed. Philadelphia: Elsevier Saunders;1425–1452.
2. Ministry of Health and Welfare. Ministry of Health and Welfare [homepage]. c2011. cited 2011 July 2. Seoul: Ministry of Health and Welfare;Available from: http://www.mw.go.kr.
3. Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998. 18:605–619.
4. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006. 59:407–412.
5. Samejima M, Tamura S, Kodama T, Yuuki Y, Takasaki J, Sekiva R, et al. Pulmonary complication following intra-arterial infusion of lipiodol-adriamycin emulsion for hepatocellular carcinoma, report of a case. Nippon Igaku Hoshasen Gakkai Zasshi. 1990. 50:24–28.
6. Kita T, Tsuji H, Takazakura E. Abnormalities in pulmonary perfusion scans after transcatheter arterial embolization of the liver. Nihon Kyobu Shikkan Gakkai Zasshi. 1996. 34:413–421.
7. Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993. 187:689–693.
8. Tajima T, Honda H, Kuroiwa T, Yabuuchi H, Okafuji T, Yosimitsu K, et al. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002. 13:893–900.
9. Shiah HS, Liu TW, Chen LT, Chang JY, Liu JM, Chuang TR, et al. Pulmonary embolism after transcatheter arterial chemoembolization. Eur J Cancer Care (Engl). 2005. 14:440–442.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download