Abstract
Background
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, gefitinib and erlotinib, are effective therapies for non-small cell lung cancer (NSCLC) patients whose tumors harbor somatic mutations in EGFR. The mutations are, however, only found in about 30% of Asian NSCLC patients and all patients ultimately develop resistance to these agents. Ionizing radiation has been shown to induce autophosphorylation of EGFR and activate its downstream signaling pathways. In the present study, we have tested whether the effect of gefitinib treatment can be enhanced after ionizing radiation.
Methods
We compared the PC-9 and A549 cell line with its radiation-resistant derivatives after gefitinib treatment with cell proliferation and apoptosis assay. We also analyzed the effect of gefitinib after ionizing radiation in PC-9, A549, and NCI-H460 cells. Cell proliferation was determined by MTT assay and induction of apoptosis was evaluated by flow cytometry. Caspase 3 activation and PARP cleavage were evaluated by western blot analysis.
Results
PC-9 cells having mutated EGFR and their radiation-resistant cells showed no significant difference in cell viability. However, radiation-resistant A549 cells were more sensitive to gefitinib than were their parental cells. This was attributable to an increased induction of apoptosis. Gefitinib-induced apoptosis increased significantly after radiation in cells with wild type EGFR including A549 and NCI-H460, but not in PC-9 cells with mutated EGFR. Caspase 3 activation and PARP cleavage accompanied these findings.
Gefitinib and erlotinib, which are epidermal growth factor receptor (EGFR) inhibitors, are useful for treating advanced non-small cell lung cancer patients. However, patients who can benefit from these EGFR inhibitors are limited to about 30% of the total non-small cell lung cancer cases having mutation of EGFR gene1. Moreover, even the lung cancers responsive to the agents eventually develop resistance due to the appearance of secondary EGFR mutations or an activated c-Met signal transduction pathway which is a bypass2.
As for radiation therapy, combination with chemotherapy is positioned as a standard treatment for locally advanced non-small cell lung cancer in stage IIIB. Despite, the current combination therapy still has unsatisfactory treatment results, which often lead to systemic chemotherapy to treat recurrence3.
There have been trials to improve treatment effects by combining these EGFR inhibitors with radiation therapy. One of the examples is the multinational randomized study on the combination of irradiation with cetuximab administration for locally advanced squamous cell carcinoma of the head and neck, which contributed to extended survival periods4. In several animal experiments, EGFR inhibitors were reported to enhance sensitivity of lung cancer cells on radiation5-8, but no independent report on enhanced treatment results from combination therapy of irradiation and administration of EGFR inhibitors has been released yet in lung cancer patients.
According to numbers of studies, EGFR pathways are activated when cancer cells are irradiated, and RAS/mitogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K)/AKT pathway are also activated and accordingly, cell proliferation occurs to resist against radiation9-12. If EGFR pathways are more activated than the ground state upon irradiation, EGFR inhibitors may be administered at this point of time with an expectation of enhanced effects. Therefore, we developed a hypothesis; if EGFR inhibitors are administered when EGFR pathways are activated upon irradiation, the effects of the agents will enhance. We tried to verify the hypothesis using lung cancer cells.
Lung cancer cell strains of A549 and NCI-H460 were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA). The PC-9 cell strain having exon 19 deletion of the EGFR gene was provided by F. Koizumi and K. Nishio (National Cancer Center Hospital, Tokyo, Japan). Each cell strain was cultured using the RPMI1640 culture media containing 10% fetal bovine serum and 1% gentamicin sulfate in a CO2 cell incubator (37℃, 5% CO2). Gefitinib, an EGFR inhibitor, was provided by AstraZeneca Korea (Seoul, Korea). Methylthiazol-2-yl-2, 5-diphenyl-tetrazolium bromide (MTT) and propidium iodide (PI) were purchased from Sigma (St. Louis, MO, USA), and annexin V-FITC was purchased from BD Bioscience (San Jose, CA, USA). Protein assay kit for protein quantification was purchased from Bio-Rad (Richmond, CA, USA). Antibody against caspase-3, and secondary antibody were purchased from Cell Signaling (Boston, MA, USA), and antibody against p-EGFR, p-Akt, p-ERK, PARP and β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence (ECL) kit was purchased from PerkinElmer (Waltham, MA, USA).
A 2 Gy of gamma ray was radiated to each of A549 and PC-9 cells using Cs-137 cell radiator (Atomic Energy of Canada Ltd, Canada). For establishing resistance, A549 and PC-9 cells had survived from the 2 Gy of gamma irradiation were cultured up to 80% confluence. Cells were repeatedly irradiated with escalating dose of gamma ray and finally, cells were exposed with 6 Gy for 6 months or more to be established as resistant cell lines.
6×103 of cells were seeded in a 96-well plate and cultured for 12 hours or more. And then, gefitinib was treated by each concentration for 72 hours. Three hours after MTT reagent was applied to each plate, 10% of sodium dodecyl sulfate solution was added to dissolve violet formazan generated by living cells. After 24-hour incubation, results were analyzed at 595 nm using microplate reader (Bio-Rad; Richmond, CA, USA).
A total of 4×105 cells were cultured on a 60 mm dish one day before gefitinib treatment, and cells were collected after 48 hours. Each cell was treated with annexin V binding buffer (150 mM NaCl, 18 mM CaCl2, 10 nM HEPES, 5 mM KCl, 1 mM MgCl2) and then, reacted with annexin V (1 g/mL) and 50 g/mL propidium iodide (PI) at a dark place for 30 minutes. After then, fluorescence-activated cell sorting (FACS) was conducted and analyzed using CellQuest software (BD Biosciences; Franklin Lakes, NJ, USA).
In order to confirm changes in cell death and EGFR-related signaling molecules, cultured cells were collected and dissolved in lysis buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 10 mM NaF, 300µM p-nitrophenyl phosphate, 1µg/mL leupeptin, 1 mM PMSF, 10 µg/mL aprotinin, pH 7.3) and then, centrifuged and quantified. Using same amount of protein, western blot was done with 10% or 12% SDS-PAGE gel. After electrophoresis, gel was moved to nitrocellulose membrane, and soaked in TBST (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.01% Tween-20) solution containing 5% nonfat skim milk for an hour at room temperature. Each of the primary antibodies was diluted to the ratio of 1:1,000 and reacted over night. Membranes which completed reaction were washed 3 times with TBST and then, reacted with the secondary antibody for about an hour and washed 3 times with TBST. The washed membranes were developed using the ECL kit.
To begin with, we authors used established radiation-resistant cell lines which have been kept for experiments. Gefitinib was administered by each concentration to radiation-resistant cell lines which were repeatedly exposed to radiation and to their parental cells and then, survival rates were calculated through MTT analysis 72 hours later. In case of PC-9 cells having exon 19 deletion of EGFR gene, no significant difference of survival was observed between parental cells and radiation-resistant cells upon administration of gefitinib. By contrast, radiation-resistant A549 cells, not having mutant EGFR genes, showed significant reduction in survival rates compared with parental cells upon administration of gefitinib. Half maximal inhibitory concentration (IC50) of A549 parental cells were 6.10µM, and IC50 of radiation-resistant cells was 0.008µM showing a significant reduction (Figure 1A).
Additionally, 48 hours after gefitinib administration, FACS was conducted using annexin V and PI and apoptosis was analyzed. In case of PC-9 cells, apoptosis fractions induced after administering different concentrations of gefitinib showed no difference between the parental cells and the radiation-resistant cells. By contrast, in case of A549 cells, upon administering 0.1µM of gefitinib, parental cells and radiation-resistant cells showed 4.64% and 45.06% of apoptosis fractions respectively, and with 1µM of gefitinib, 6.52% and 43.13% respectively, showing significant difference likewise cell survival rates (p<0.05) (Figure 1B).
Each of PC-9, A549, NCI-H460 cells was irradiated with 2 Gy and after 48 hours, 1µM, 20µM and 30µM of gefitinib, being equivalent to IC10~IC20, were administered. FACS analysis was conducted 48 hours after the administrations to measure apoptosis fraction.
In case of PC-9 cells, gefitinib-induced apoptosis fractions were 15.19% without previous radiation, and 16.81% with previous radiation, showing no significant difference between the treatments. By comparison, A549 cells showed 14.16% and 23.06% (p<0.05) with the respective treatment, and NCI-H460 cells showed 13.12% and 19.16% (p<0.05) with the respective treatment, showing significant differences (Figure 2A). The difference of gefitinib-induced apoptosis fractions between the treatment with previous radiation and the treatment without previous radiation showed 1.62% with PC-9 cells, 8.90% with A549 cells, and 6.04% with NCI-H460 cells (p<0.05) (Figure 2B).
In order to confirm whether the changes observed in the above experiments were also found in apoptosis-related proteins or not, western blot was conducted 24 hours after administration of gefitinib. According to the experiments using radiation-resistant cells and their parental cells, no significant difference in reduction of procaspase 3, increase in caspase 3 active form and PARP cleavage, which are expected to occur upon administration of gefitinib, was found between radiation-resistant and parental cells in case of PC-9 cells. By contrast, in case of A549 cells, radiation-resistant cells showed comparatively more changes of decrease in procaspase 3, increase in caspase 3 active form and PARP cleavage than parent cells did upon administration of gefitinib (Figure 3A).
In the experiment of gefitinib treatment after radiation, PC-9 cells showed no significant difference between the treatment without previous radiation and the treatment with previous radiation, but A549 cells showed significant changes of decrease in procaspase 3, increase in caspase 3 active form and PARP cleavage in case of the gefitinib treatment with previous radiation compared with that without previous radiation (Figure 3B).
Based on the above results, EGFR wild-type A549 and NCI-H460 cells showed a significantly enhanced effect of gefitinib after irradiation. However, PC-9, having EGFR mutation, did not show these results. This is interesting results which have not been expected. It has been well known that wild-type cells without EGFR mutation generally show a resistance against EGFR inhibitors. If effects of EGFR inhibitors significantly increase after irradiation, we could expect effects of EGFR inhibitors even in these EGFR wild type cells.
A number of studies reported activated EGFR pathways upon irradiation on cancer cells resulting in cell proliferation and resistance against radiation effects. Mechanisms involved in the above are firstly, swift repopulation through activated RAS/MAPK/ERK pathways after the irradiation9,13-16 and secondly, increased cell survival due to activated PI3K/AKT pathways17,18. Thirdly, a hypothesis explains that EGFR directly involves in repairing radiation-caused DNA damage as a mediator. According to studies, when A549 cells were irradiated, EGFR moves into nucleus to combine with protein kinase (DNA-PK) which is essential for repairing DNA damaged by radiation10.
In other words, this implies that activated EGFR pathways upon irradiation possibly functions as a mechanism of cancer cell survival. The fact that the EGFR pathway is activated compared with a ground state after irradiation may become a base to setup a hypothesis explaining increased responses to EGFR inhibitors. However, further studies are necessary to explain the reason why no same result is shown in PC-9 cells with EGFR mutation. As one of the possible explanations, PC-9 cells with EGFR mutation already have sufficiently activated EGFR pathways and accordingly, additional activation stimulation by irradiation may not result in much difference.
In the present study, radiation-resistant cell lines which were already exposed to radiation were compared with their parental cells. Meanwhile, to find out effects of gefitinib, the agent was administered to each of the irradiated and non-irradiated cells. In both experiments, they showed the same results, even though the degree of irradiation was much different from each other experiment.
Most of the studies on EGFR inhibitors and radiation therapy are focused on finding out a radiosensitizer which can improve the treatment effect of radiation therapy by pre-processing EGFR inhibitors5-8,19-24. There is an example of multinational randomized study reporting a statistically significant increase in survival periods by combining cetuximab with radiation therapy for treating locally advanced squamous cell carcinoma of head and neck. According to the above study, a group of single treatment with 213 participants and a group of combined treatment with 211 participants showed a statistically significant difference with 14.9 months and 24.4 months respectively in terms of the duration of locoregional control (p=0.005). In addition, the overall survival periods were 29.3 months and 49.0 months respectively, and the progression-free survival periods were 12.4 months and 17.1 months respectively, which were also statistically significant4. Being different from the results of the above study, the present study showed that previous radiation can change the effects of EGFR inhibitors which were administered following irradiation. In conclusion, the significance of the present study is suggesting that irradiation may increase the effects of EGFR inhibitors.
Figures and Tables
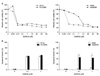 | Figure 1(A) Effect of gefitinib on proliferation determined by using the MTT assay. Cells were treated with the indicated concentrations of gefitinib and proliferation was determined 72 hours later. PC-9 cells having mutated EGFR and their radiation-resistant cells (PC-9/RR) showed no significant difference in cell viability. However, radiation-resistant A549 cells (A549/RR) were more sensitive to gefitinib than were their parental cells. (B) Induction of apoptosis after gefitinib treatment for 48 hours. After staining with annexin V and PI, the cells were analyzed by flow cytometry to analyze apoptosis. PC-9 cells and their radiation-resistant cells (PC-9/RR) showed a similar apoptosis fraction at each concentration. However, radiation-resistant A549 cells (A549/RR) showed a significantly higher rate of apoptosis than did their parental cells. *p<0.05, †p<0.05. |
 | Figure 2(A) Induction of apoptosis by gefitinib treatment in PC-9, A549, NCI-H460 cells. The cells were irradiated with 2 Gy and treated with each concentration of gefitinib (1 µM for PC-9, 20 µM for A549, 30 µM for NCI-H460) after 48 hours. A further 48 hours after the gefitinib treatment, apoptosis was analyzed by FACS. The results were compared to those of cells treated with only gefitinib without previous irradiation. G, gefitinib; RT, radiation. *p<0.05, †p<0.05. (B) The difference of apoptosis induction by gefitinib with or without previous irradiation was plotted. Gefitinib-induced apoptosis increased after radiation in cells with wild type EGFR, including A549 and NCI-H460, but not in PC-9 cells with mutated EGFR. *p<0.05, †p<0.05. |
 | Figure 3Western blot analysis of procaspase 3, caspase 3, and PARP. (A) PC-9 radiation-resistant cells (PC-9/RR), A549 radiation-resistant cells (A549/RR) and their parental cells were exposed to gefitinib for 24 hours in each concentration and were harvested for the results. Actin served as a loading control. Gefitinib-induced apoptosis increased in A549/RR cells compared to their parental cells. (B) PC-9 and A549 cells were either irradiated or not irradiated with 2 Gy and treated with gefitinib (1 µM for PC-9, 20 µM for A549) after 48 hours. Twenty four hours after gefitinib treatment, the cells were harvested. Gefitinib-induced apoptosis increased after irradiation in A549 but not in the PC-9 cells. C: control; G: gefitinib; RT: radiation. |
Acknowledgements
This work was supported by a grant (50452-2011) from the Korea Institute of Radiological and Medical Sciences Research Fund (RTR11-02).
References
1. Jänne PA, Johnson BE. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2006. 12:4416s–4420s.
2. Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008. 14:2895–2899.
3. Haasbeek CJ, Slotman BJ, Senan S. Radiotherapy for lung cancer: clinical impact of recent technical advances. Lung Cancer. 2009. 64:1–8.
4. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006. 354:567–578.
5. She Y, Lee F, Chen J, Haimovitz-Friedman A, Miller VA, Rusch VR, et al. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 selectively potentiates radiation response of human tumors in nude mice, with a marked improvement in therapeutic index. Clin Cancer Res. 2003. 9:3773–3778.
6. Park JS, Jun HJ, Cho MJ, Cho KH, Lee JS, Zo JI, et al. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin Cancer Res. 2006. 12:4989–4999.
7. Shibuya K, Komaki R, Shintani T, Itasaka S, Ryan A, Jurgensmeier JM, et al. Targeted therapy against VEGFR and EGFR with ZD6474 enhances the therapeutic efficacy of irradiation in an orthotopic model of human non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007. 69:1534–1543.
8. Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008. 14:1266–1273.
9. Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997. 15:1191–1197.
10. Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005. 280:31182–31189.
11. Das AK, Sato M, Story MD, Peyton M, Graves R, Redpath S, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006. 66:9601–9608.
12. Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, et al. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007. 67:5267–5274.
13. Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Amir C, Dent P, et al. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999. 18:4756–4766.
14. Suy S, Anderson WB, Dent P, Chang E, Kasid U. Association of Grb2 with Sos and Ras with Raf-1 upon gamma irradiation of breast cancer cells. Oncogene. 1997. 15:53–61.
15. Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988. 27:131–146.
16. Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003. 159:283–300.
17. Contessa JN, Hampton J, Lammering G, Mikkelsen RB, Dent P, Valerie K, et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002. 21:4032–4041.
18. Toulany M, Dittmann K, Kruger M, Baumann M, Rodemann HP. Radioresistance of K-Ras mutated human tumor cells is mediated through EGFR-dependent activation of PI3K-AKT pathway. Radiother Oncol. 2005. 76:143–150.
19. Huang SM, Li J, Armstrong EA, Harari PM. Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa). Cancer Res. 2002. 62:4300–4306.
20. Ochs JS. Rationale and clinical basis for combining gefitinib (IRESSA, ZD1839) with radiation therapy for solid tumors. Int J Radiat Oncol Biol Phys. 2004. 58:941–949.
21. Burdak-Rothkamm S, Rübe CE, Nguyen TP, Ludwig D, Feldmann K, Wiegel T, et al. Radiosensitivity of tumor cell lines after pretreatment with the EGFR tyrosine kinase inhibitor ZD1839 (Iressa). Strahlenther Onkol. 2005. 181:197–204.
22. Colquhoun AJ, Mchugh LA, Tulchinsky E, Kriajevska M, Mellon JK. Combination treatment with ionising radiation and gefitinib ('Iressa', ZD1839), an epidermal growth factor receptor (EGFR) inhibitor, significantly inhibits bladder cancer cell growth in vitro and in vivo. J Radiat Res (Tokyo). 2007. 48:351–360.
23. Harari PM. Stepwise progress in epidermal growth factor receptor/radiation studies for head and neck cancer. Int J Radiat Oncol Biol Phys. 2007. 69:2 Suppl. S25–S27.
24. Thariat J, Yildirim G, Mason KA, Garden AS, Milas L, Ang KK. Combination of radiotherapy with EGFR antagonists for head and neck carcinoma. Int J Clin Oncol. 2007. 12:99–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download