Abstract
Imatinib mesylate, a selective inhibitor of BCR-ABL kinase activity, has demonstrated significant clinical efficacy in the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). It has become the standard of treatment for these diseases. Although the toxicity profile of imatinib is superior to that of interferon or other cytotoxic agents, some adverse events including edema, gastrointestinal toxicities and hematologic toxicities are commonly observed in the patients treated by imatinib. We present two cases of imatinib induced interstitial pneumonitis during the treatment of a chronic phase of CML.
Chronic myeloid leukemia (CML) is a unique disease with a t(9;22) reciprocal chromosomal dislocation, which results in a aberrant gene formation of BCR-ABL1. Though most patients with CML are diagnosed in chronic phase with little symptoms, the natural course of this disease is fatal with progression to blast crisis2.
Before the era of targeting agent of CML, allogenic stem cell transplantation (SCT) was the only way to induce durable remissions. However allogenic SCT has a substantial mortality rate and a significant proportion of patients without matched donor were not able to be treated with this treatment option. The development of agents targeting BCR-ABL such as imatinib mesylate (Gleevec® or Glivec®; Norvatis, Basel, Switzerland) improved the prognosis dramatically3. The International randomized study of interferon and STI571 trial (IRIS trial) demonstrated that long-term estimated rates of freedom from progression to accelerated or blast phase were more than 90% with imatinib therapy4. Standard dose of imatinib therapy is now considered to the standard treatment for the patient with newly diagnosed CML.
Though imatinib is generally well tolerated with rare grade 3 or 4 non-hematologic toxicities, adverse events including myalgia and fluid retention were reported5. Some patients complain of dyspnea during their treatment of imatinib. Most of the cases are related to pulmonary edema due to fluid retention. However, the cases of imatinib-induced interstitial pneumonitis were reported sporadically as a rare adverse event6-9. In Korea, only one case of interstitial pneumonitis was reported during imatinib therapy for GIST10.
We report here 2 cases of imatinib-induced interstitial pneumonitis with one irreversible case.
A 68-year-old man was diagnosed to a chronic phase of Philadelphia chromosome-positive CML in September 2008. He was treated with imatinib mesylate at a dose 400 mg per day. A complete cytogenetic response (CCyR) was achieved after 3 months of therapy. At that time, he presented with cough, sputum, and dyspnea. Chest X-ray showed bilateral diffuse haziness in both lower lung fields (Figure 1A).
After 4 months of therapy, he was admitted due to aggravation of dyspnea. His blood pressure, heart rate, body temperature and respiratory rates were 140/90 mm Hg, 80/min, 37.4℃ and 20/min, respectively. On physical exam, there was no remarkable finding except crackles in both lower lung fields. Pulmonary function test revealed pulmonary restrictive pattern with forced vital capacity (FVC) of 2.16 L (56% predicted). High resolution computed tomography of chest showed bilateral diffuse subpleural nodules, interlobular septal thickening, reticulation and peribronchial ground glass opacities in both lungs, suggesting diffuse interstitial lung disease (Figure 1B). Ultrasound-guided percutaneous needle biopsy obtained in left lower lung field showed septal edema with fibrosis and infiltration of mixed inflammatory cells (Figure 2). Though his poor general condition didn't allow us perform surgical biopsy for further evaluation, the above percutaneous needle biopsy findings were compatible with drug-induce interstitial pneumonitis. Moreover, at that time, imatinib was the only medicine he was taking.
With the above data, he was diagnosed to an imatinib-induced pulmonary interstitial pneumonitis. Imatinib was discontinued and steroid therapy with prednisone (1 mg/kg/day) was started. After 20 days of steroid therapy, his dyspnea was progressed and mechanical ventilator support was applied. Even with mechanical ventilator support, his respiratory function was worsened and finally he was expired 30 days after admission.
A previously healthy 71-year-old man was diagnosed to a chronic phase of Philadelphia chromosome-positive CML on February 2009. Imatinib mesylate was initiated at a dose of 400 mg daily. A complete cytogenetic response (CCyR) was achieved after 3 months of therapy.
At that time, he presented with dyspnea on exercise. His blood pressure, heart rate, body temperature and respiratory rates were 120/80 mm Hg, 82/min, 36℃ and 20/min, respectively. His physical examination revealed crackles in both lower lung fields.
Pulmonary function test revealed pulmonary restrictive pattern with forced vital capacity (FVC) of 2.29 L (63% predicted). Chest X-ray showed patchy nodular ground glass opacity infiltration in both lower lung fields (Figure 3A). Computed tomography of chest showed diffuse poorly defined ground glass opacity infiltrations, along axial interstitial and interlobular septum, with lower lung zone dominance, compatible with interstitial pneumonitis (Figure 3B). Bronchoalveolar lavage (BAL) was performed in right middle lobe bronchus, BAL cell count and differential was RBC 150/mm3, WBC 170/mm3 (macrophages 28%, lymphocytes 77%, eosinophils 6%). This pattern can be showed at drug induced interstitial pneumonitis. A wedge resection biopsy by Video-Assisted Thoracic Surgery (VATs) performed on left lower lobe showed pneumonic consolidation with inflammatory cell infiltration and collagenous fibrosis (Figure 4).
Imatinib was discontinued and steroid therapy with prednisone 1 mg/kg/day was started under the diagnosis of imatinib-induced interstitial pneumonitis. Two weeks after steroid therapy, his dyspnea and cough was improved. Prednisone was tapered down over 2 weeks. A follow-up chest computed tomography (CT) scan 2 months later showed improvement in the appearance of the previously described pulmonary findings. However, restrictive pattern of pulmonary function test still remained (FVC 2.17 L, 60% predicted; FEV1 2.11 L, 86% predicted; and FEV1/FVC 97%).
He was treated with Nilotinib 800 mg daily instead of Imatinib mesylate.
Interstitial pneumonitis is a rare adverse event of imatinib therapy. The diagnosis of imatinib-induced interstitial pneumonitis is usually made by the compatible history and clinical findings6-10. The patients with this disease complained of various respiratory symptoms including dyspnea, cough and hypoxia. The findings of chest radiographs and CT are also quite variable including hypersensitivity pattern, interstitial pneumonitis pattern, cryptogenic organizing pneumonia pattern, nodular pattern and peribronchovascular bundle pattern7. Histopathologic diagnosis by trans-bronchial lung biopsy or video-assisted thoracoscopic surgery (VATS) was performed only in a small proportion of patients. The histopathologic findings are usually fibrosis and inflammatory cell infiltration7.
Most patients with interstitial pneumonitis are recovered after withdrawal of imatinib with or without steroid therapy. However, a number of patients with this adverse event did not improved even after withdrawal of imatinib7,9. We experienced one case of irreversible interstitial pneumonitis by imatinib therapy (case 1). The risk factors as well as the prognostic factors of interstitial pneumonitis after imatinib therapy are unknown. Some authors suggest that long-term use of imatinib can make interstitial pneumonitis irreversible9. In our case 1, imatinib was prescribed for about 1 month after his respiratory symptom was appeared. It is possible that the failure from early withdrawal of imatinib made the outcome of this patient grave, whereas other patient with early withdrawal of imatinib was fully recovered. Though it is impossible to make a conclusion with above two cases, early diagnosis and early withdrawal of imatinib may be a key element in imatinib-induced interstitial pneumonitis.
Though the incidence of imatinib-induced interstitial pneumonitis is known to be less than 2% of patients, a considerable number of this adverse events were reported in Japan7. Many cases of interstitial pneumonitis were reported too in Japan by gefitinib, a tyrosine kinase inhibitor for treatment of non-small cell lung cancer11. The incidence of this pulmonary adverse event may be different by ethnics, and Asian population may be more vulnerable.
In both of our cases, interstitial pneumonitis was developed after 3 months of imatinib therapy. The median duration until development of interstitial pneumonitis was 49 days in a report of 27 cases7. Though the duration of imatinib therapy before development of interstitial pneumonitis is quite variable, a careful history focusing to the respiratory symptoms and chest X-ray should be recommended after 3 months of imatinib therapy. A prompt withdrawal of imatinib should be considered in the patients with the respiratory symptoms and/or abnormal chest X-ray findings.
Figures and Tables
Figure 1
(A) Chest PA X-ray shows diffuse subpleural haziness in both lung fields. (B) Chest CT shows bilateral diffuse subpleural nodules, interlobular septal thickening, reticulation and peribronchial ground glass opacities in both lungs. CT: computed tomography.
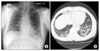
Figure 2
(A) The needle biopsied lung tissue shows pneumonic consolidation. The solid portion shows pale-pinkish fibroblastic proliferation (arrow) and scanty inflammatory infiltrate (H&E stain, ×40). (B) The alveolar septum is uniformly fibrous-thickened and ball like smooth muscle hyperplasia (arrow) (H&E stain, ×100). (C) The alveoli are partly desquamated and replaced by type II pneumocytes (arrow). The alveolar spaces are filled with a few lymphocytes and eosinophils (H&E stain, ×200). (D) Masson Trichrome staining contrasts a collagenous fibrosis from surrounding fibrinous component (Masson Trichrome stain, ×100).
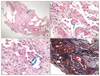
Figure 3
(A) Chest X-ray shows patchy nodular ground glass opacity infiltrations in both lower lung fields. (B) Chest CT shows bilateral diffuse poorly defined ground glass opacity infiltrations, along axial interstitial and interlobular septum, with lower lung zone dominance. CT: computed tomography.
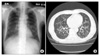
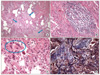
Figure 4
(A) The wedge-resected lung shows a temporally uniform pneumonic consolidation with pale pinkish young fibroblastic proliferation (arrows) with many mixed inflammatory infiltrate. Some alveoli are destroied with honey combing (H&E stain, ×40). (B) Patchy mononuclear infiltrate is identified, predominantly lymphocytes (H&E stain, ×100). (C) Type II pneumocyte hyperplasia and multinucleated giant cells (circle) are identified (H&E stain, ×200). (D) The masson-Trichrome staining contrasts collagenous fibrosis (blue) background of fibrinous exudate (dark-red) (Masson Trichrome stain, ×100).
References
1. Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000. 96:3343–3356.
2. Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007. 7:441–453.
3. Pavlovsky C, Kantarjian H, Cortes JE. First-line therapy for chronic myeloid leukemia: Past, present, and future. Am J Hematol. 2009. 84:287–293.
4. Guilhot F, Druker B, Larson RA, Gathmann I, So C, Waltzman R, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009. 94:1669–1675.
5. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001. 344:1031–1037.
6. Ma CX, Hobday TJ, Jett JR. Imatinib mesylate-induced interstitial pneumonitis. Mayo Clin Proc. 2003. 78:1578–1579.
7. Ohnishi K, Sakai F, Kudoh S, Ohno R. Twenty-seven cases of drug-induced interstitial lung disease associated with imatinib mesylate. Leukemia. 2006. 20:1162–1164.
8. Rosado MF, Donna E, Ahn YS. Challenging problems in advanced malignancy: case 3. Imatinib mesylate-induced interstitial pneumonitis. J Clin Oncol. 2003. 21:3171–3173.
9. Seki N, Ito A, Watanabe K, Shibakuki R, Seto T, Uematsu K, et al. Irreversible imatinib-induced pneu monitis following long-term imatinib administration. Intern Med. 2007. 46:1941–1942.
10. Lee JW, Kim HJ, Kim KJ, Shin KC, Hong YH, Chung JH, et al. A case of imatinib-mesylate associated hypersensitivity pneumonitis. Tuberc Respir Dis. 2005. 59:423–426.
11. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download