Abstract
Background:
Statins can regulate the production of pro-inflammatory cytokines and inhibit MMP production or activation in a variety of types of cells. This study evaluated whether statins would inhibit MMP release from human lung fibroblasts, which play a major role in remodeling processes.
Methods:
This study, using an in-vitro model (three-dimensional collagen gel contraction system), evaluated the effect of cytokines (tumor necrosis factor-α, TNF-a and interleukin-1β, IL-1b) on the MMP release and MMP activation from human lung fibroblasts. Collagen degradation induced by cytokines and neutrophil elastase (NE) was evaluated by quantifying hydroxyproline.
Results:
In three-dimensional collagen gel cultures (3D cultures) where cytokines (TNF-a and IL-1b) can induce the production of MMPs by fibroblasts, it was found that simvastatin inhibited MMP release. In 3D cultures, cytokines together with NE induced collagen degradation and can lead to activation of the MMP, which was inhibited by simvastatin.
REFERENCES
1.Laurell CB. Eriksson S. The electrophoretic alpha;1 globulin pattern of serum in alpha: 1 antitrypsin deficiency. Scand J Clin Lab Invest. 1963. 15:132–40.
2.Shapiro SD. The pathogenesis of emphysema: the elastase: antielastase hypothesis 30 years later. Proc Assoc Am Physicians. 1995. 107:346–52.
3.Seagrave J. Oxidative mechanisms in tobacco smoke-induced emphysema. J Toxicol Environ Health A. 2000. 61:69–78.

4.Barter MJ., Hui W., Lakey RL., Catterall JB., Cawston TE., Young DA. Lipophilic statins prevent matrix metal-loproteinase-mediated cartilage collagen breakdown by inhibiting protein geranylgeranylation. Ann Rheum Dis. 2010. 69:2189–98.

5.Newton CJ., Ran G., Xie YX., Bilko D., Burgoyne CH., Adams I, et al. Statin-induced apoptosis of vascular endothelial cells is blocked by dexamethasone. J Endo-crinol. 2002. 174:7–16.

6.Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972. 54:626–37.

7.Mio T., Adachi Y., Romberger DJ., Ertl RF., Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996. 32:427–33.

8.Gutierrez HH., Pitt BR., Schwarz M., Watkins SC., Lowenstein C., Caniggia I, et al. Pulmonary alveolar epithelial inducible NO synthase gene expression: regulation by inflammatory mediators. Am J Physiol. 1995. 268:L501–8.

9.Bergman I., Loxley R. Two improved and simplified methods for the spectrophotometric determination ofhydroxyproline. Anal Chem. 1963. 35:1961–5.
10.Edwards CA., O'Brien WD Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980. 104:161–7.

11.Kleiner DE., Stetler-Stevenson WG. Quantitative zymog-raphy: detection of picogram quantities of gelatinases. Anal Biochem. 1994. 218:325–9.

12.Zhang Y., McCluskey K., Fujii K., Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998. 161:3071–6.
13.Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979. 76:1274–8.

15.Sköld CM., Liu X., Umino T., Zhu Y., Ohkuni Y., Romberger DJ. Human neutrophil elastase augments fibroblast-mediated contraction of released collagen gels. Am J Respir Crit Care Med. 1999. 159:1138–46.

16.Zhu YK., Liu XD., Sköld CM., Umino T., Wang HJ., Spurzem JR, et al. Synergistic neutrophil elastase-cyto-kine interaction degrades collagen in three-dimensional culture. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L868–78.

17.Dans MJ., Isseroff R. Inhibition of collagen lattice contraction by pentoxifylline and interferon-alpha, -beta, and -gamma. J Invest Dermatol. 1994. 102:118–21.

18.Zhang HY., Gharaee-Kermani M., Phan SH. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1beta. J Immunol. 1997. 158:1392–9.
19.Snider GL., Failing LJ., Rennard SI. Chronic bronchitis and emphysema. Murray JF, Nadel JA, editors. editors.Texbook of respiratory medicine. 2nd ed.Philadelphia, PA: WB Saunders;1994. p. 1331–97.
20.Niewoehner DE. Anatomic and pathophysiological correlations in COPD. Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. editors.Textbook of pulmonary diseases. 6th ed.Philadelphia, PA: Lippincott-Raven;1998. p. 823–42.
21.Birkedal-Hansen H., Moore WG., Bodden MK., Windsor LJ., Birkedal-Hansen B., DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993. 4:197–250.

22.Woessner JF. The matrix metalloproteinase family. Parks WC, Mecham RP, editors. editors.Matrix metalloproteinases. San Diego, CA: Academic Press;1998. p. 1–13.

23.Tipton DA., Pabst MJ., Dabbous MK. Interleukin-1 beta-and tumor necrosis factor-alpha-independent monocyte stimulation of fibroblast collagenase activity. J Cell Biochem. 1990. 44:253–64.
24.Préaux AM., Mallat A., Nhieu JT., D'Ortho MP., Hembry RM., Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999. 30:944–50.

25.Ramos-DeSimone N., Hahn-Dantona E., Sipley J., Nagase H., French DL., Quigley JP. Activation of matrix metal-loproteinase-9 (MMP-9) via a converging plasmin/stro-melysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999. 274:13066–76.

26.Takahashi HK., Mori S., Iwagaki H., Yoshino T., Tanaka N., Weitz-Schmidt G, et al. Differential effect of LFA703, pravastatin, and fluvastatin on production of IL-18 and expression of ICAM-1 and CD40 in human monocytes. J Leukoc Biol. 2005. 77:400–7.

27.Fessler MB., Young SK., Jeyaseelan S., Lieber JG., Arndt PG., Nick JA, et al. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med. 2005. 171:606–15.

28.Lee JH., Lee DS., Kim EK., Choe KH., Oh YM., Shim TS, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005. 172:987–93.

29.Fukumoto Y., Libby P., Rabkin E., Hill CC., Enomoto M., Hirouchi Y, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001. 103:993–9.

30.Bellosta S., Via D., Canavesi M., Pfister P., Fumagalli R., Paoletti R, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998. 18:1671–8.

31.Inoue I., Goto S., Mizotani K., Awata T., Mastunaga T., Kawai S, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci. 2000. 67:863–76.
Figure 1.
Effect of Simvastatin on Three-dimensional Collagen Gel (3D-gel) Contraction in the Presence or Absence of Cytokines and Neutrophil Elastase. Gels were released into tissue culture dishes containing 5 mL of serum-free DMEM with or without simvastatin (5 mM). After 1 hour incubation, cytokines (TNF-a 5 ng/mL, IL-1b 5 ng/mL) and NE (20 nM) were added in to the gel floating medium. Vertical axis presents gel size as % of initial area, and horizontal axis presents time after release (days). The data presented are mean±SEM from three separate experiments, each of which included triplicate gels for each condition. When simvastatin added together with the combination of the neutrophil elastase and cytokines, simvastatin (5μM) significantly inhibit collagen gel contraction induced by the combination of the neutrophil elastase and cytokines. ∗p<0.01 compared with combination of neutrophil elastase and cytokines. CK: cytokines (TNF-α+IL-1b); NE: neutrophil elastase. Statin: simvastatin; SEM: standard error of the mean.
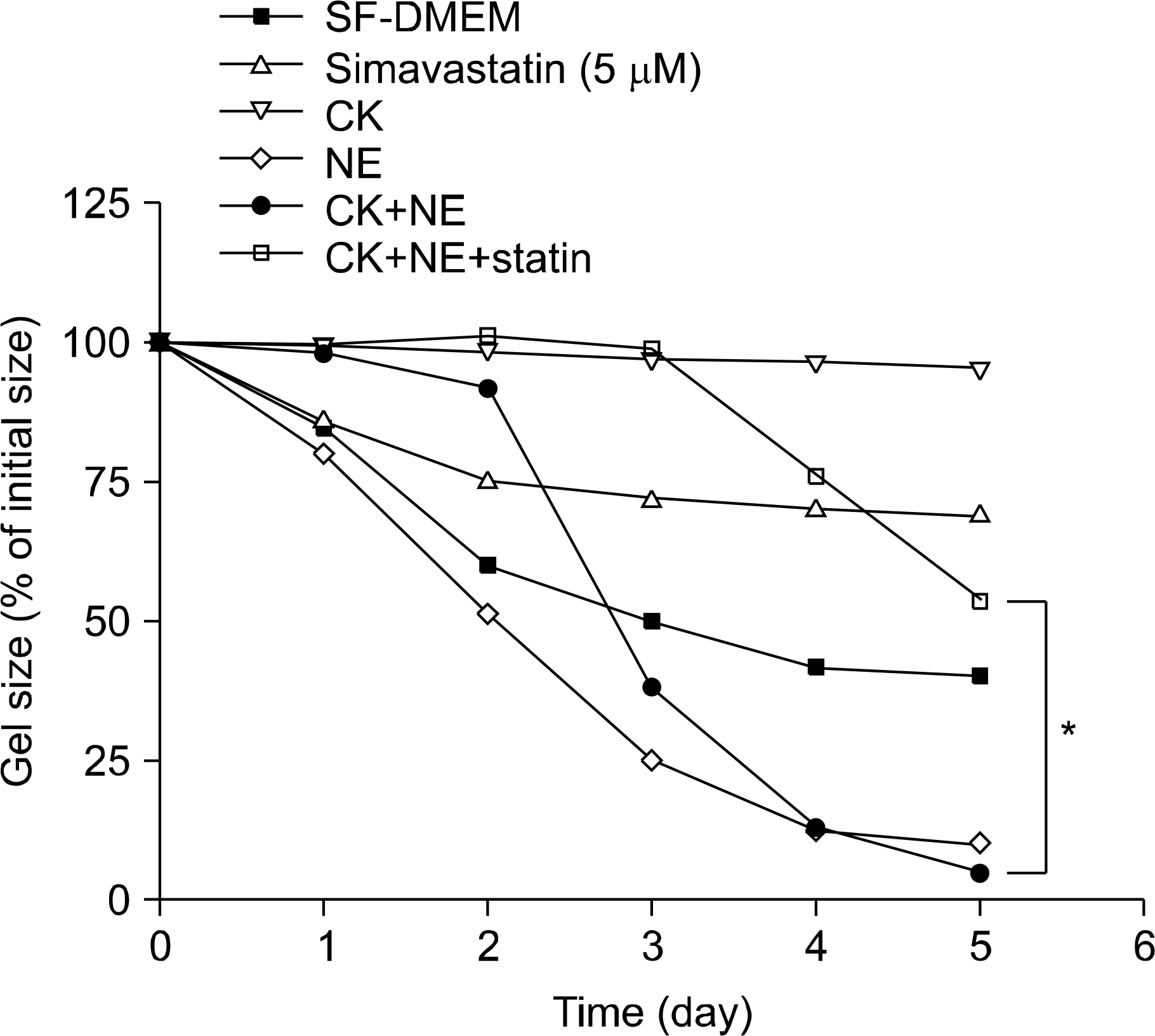
Figure 2.
Effect of Simvastatin on Collagen Gel Degradation Induced by Cytokines and NE. Gels were prepared and cultured as shown above in figure 1. On day 5, gels were harvested and subjected to hydroxyproline assay. Vertical axis presents hydroxyproline (% of control), and horizontal axis presents culture conditions. The data presented are mean±SEM from three separate experiments, each per formed in duplicate. Cytokines (TNF-a 5 ng/mL, IL-1b 5 ng/mL) alone or NE (20 nM) alone slightly decreased the hydroxyproline content. The combination of cytokines and NE, however, resulted in a significant decrease of hydroxyproline content in collagen gels. Simvastatin (5μM) significantly blocked collagen gel degradation induced by cytokines plus neutrophil elastase. ∗p <0.01 compared with combination of neutrophil elastase and cytokines, †p<0.01 compared with Cytokines alone or NE, ‡p<0.01 compared with control cultures. CK: cytokines (TNF-a+IL-1b); NE: neutrophil elastase; SEM: standard error of the mean.
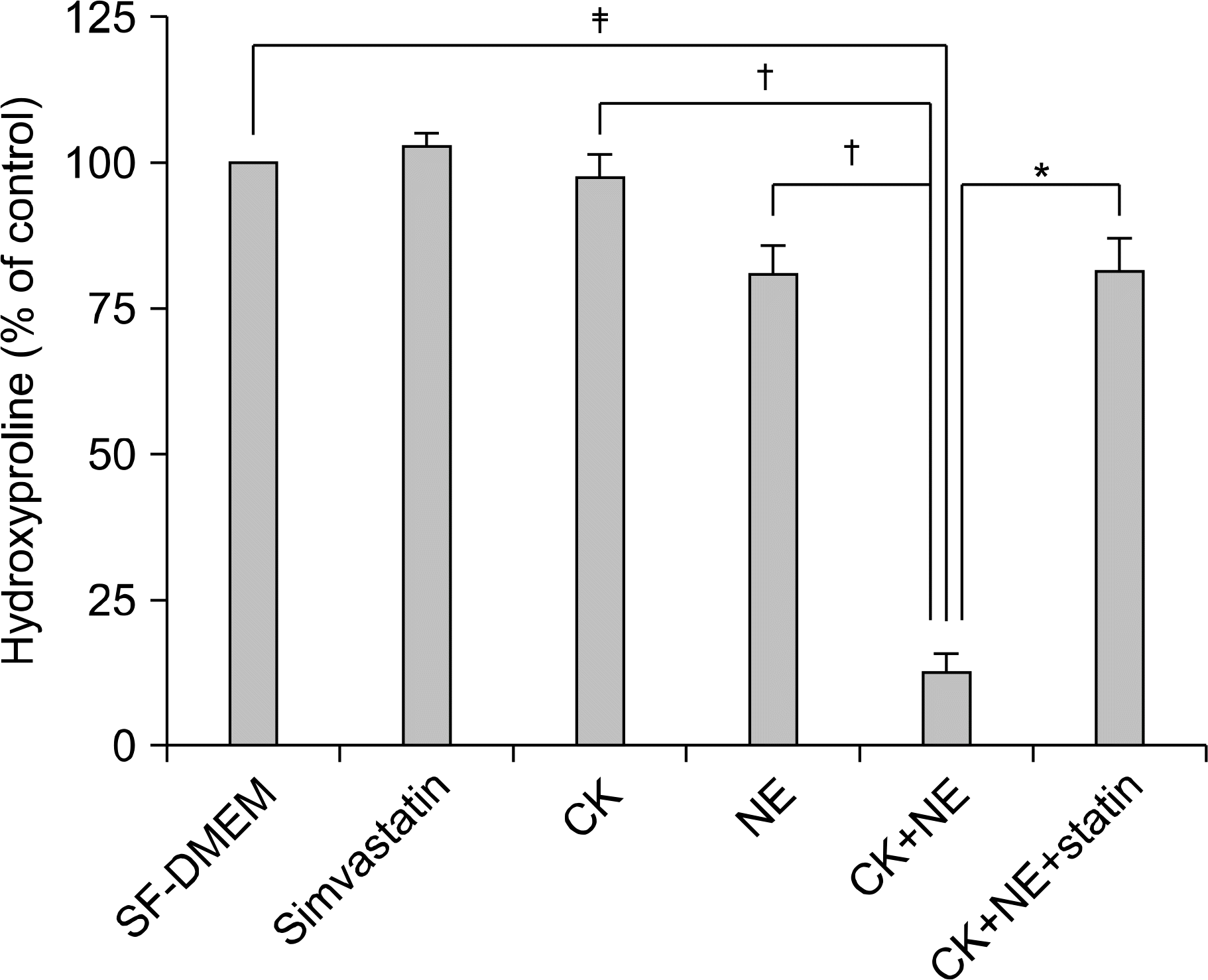
Figure 3.
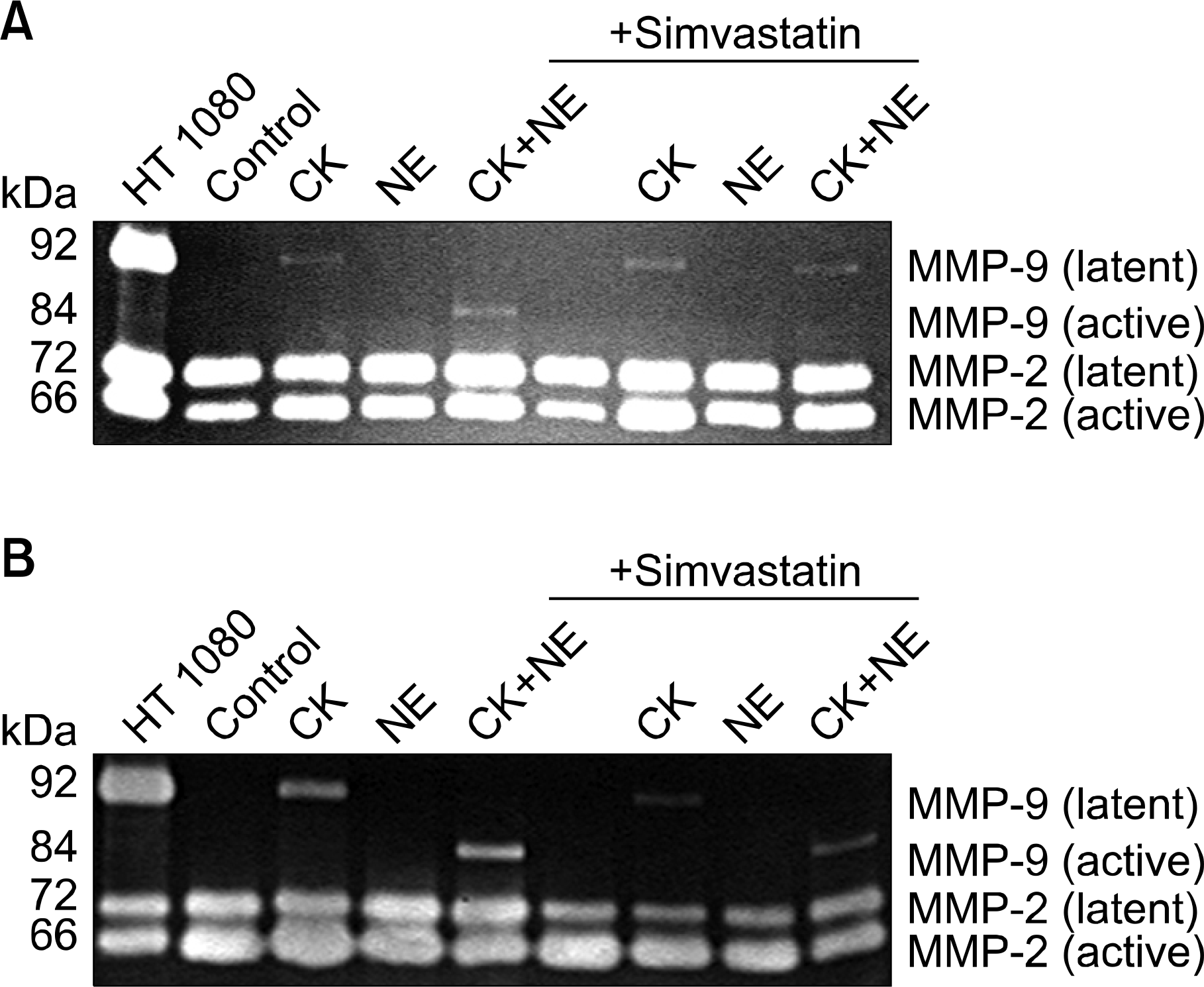
Effect of simvastatin on MMP-2 and -9 production and activation by the combination of cytokines and NE. Gels were prepared and cultured as shown above in figure 1. On day 2 (Figure 3A) and day 5 (Figure 3B), media (500μL on day 2 and the rest on day 5) were harvested and subjected to gelatin zymography. Supernatant from HT1080 cell monolayer culture was used as a positive control. Under control conditions, HFL-1 fibroblasts cultured in three-dimensional collagen gels primarily released MMP-2 (gelatinase A) into surrounding media, as identified by its characteristic molecular weights of 72 kD (latent form) and 66 kD (active form) (Figure 3A and B). MMP-9 (gelatinase B) was not produced by HFL-1 cells under control conditions. In the presence of cytokines (TNF-a and IL-1b), however, latent form of MMP-9 (92 kD) was produced and this latent MMP-9 was converted to the active form (84 kD) by NE (10 nM, Figure 3). Simvastatin (5μM) not only significantly blocked the MMP-9 conversion from latent to active form (Figure 3A), but also inhibited MMP-9 and MMP-2 production (Figure 3B). CK: cytokines (TNF-a+IL-1b); NE: neutrophil elastase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download