Abstract
Herein, we report a case of recurrent pleural metastasis after complete resection of invasive thymoma that was successfully treated with surgical resection. Thymoma and thymic carcinoma are uncommon neoplasms derived from the epithelial cells of the thymus. Approximately 30% to 50% of thymomas are asymptomatic at the time of diagnosis. However, these cancers may present with constitutional or local pressure symptoms and sometimes with paraneoplastic syndromes, especially myasthenia gravis. Surgical resection is the mainstay of thymoma treatment and has been shown to remarkably improve long-term survival. Despite complete resection, local recurrences are frequent, and surgery is the cornerstone of therapy even in cases of recurrent thymoma. We experienced a 67-year-old male patient with pleural metastasis that developed 6 years after complete surgical resection of invasive thymoma. The pleural mass was excised by video-assisted thoracoscopic surgery. Histopathological examination revealed an invasive World Health Organization (WHO) type B2 thymoma.
Thymoma is the most common tumor that develops in the anterior mediastinum. It is usually indolent and grows slowly1. In general, although the natural history of thymoma is difficult to anticipate, malignant thymoma is often invasive and likely to relapse. Awad et al.2 reported a case of stage I thymoma that recurred at the pleura 32 years after resection. However, there has been no report of a case that has recurred after a long follow-up period in Korea. We present herein a case of thymoma that recurred at the pleura 6 years after surgical resection plus postoperative radiation therapy. A brief review of the literature has been included.
A 67-year-old man presented with abnormal findings in the pleura by chest computed tomography (CT). He underwent excision through sternotomy and postoperative radiation therapy (5,040 cGy) due to invasive thymoma 6 years before this presentation. No abnormality had been found at periodic follow-ups before a mass was detected on CT scans. At that time, he had no symptoms. A lobulated mass was detected on chest radiographs (Figure 1) 6 year before this presentation, which was resected through sternotomy. Histological examination of the surgical specimen revealed type B2 invasive thymoma. There was no remarkable history of diabetes mellitus and hypertension. He was a non-smoker and an office clerk by occupation.
On physical examination, his nutritional and general conditions were good, and blood pressure, body temperature, pulse rate and respiratory rate were normal. Hematologic tests revealed hemoglobin 13.5 g/dL, hematocrit 39.9%, ESR 26 mm/hr (normal range, 0~30 mm/hr), white blood cells 4,900/mm3, neutrophils 63%, lymphocytes 24%, monocytes 9%, and eosinophils 2%. Blood chemistry tests exhibited serum albumin 3.9 g/dL, serum calcium 8.6 mm/dL, AST/ALT 21/12 U/L, serum urea nitrogen 1.8 mg/dL, serum creatinine 1.1 mg/dL, and C-reactive protein 0.19 mg/dL. Pulmonary function tests showed FVC 3.19 L (90% of predicted value), FEV1 2.06 L (83% of predicted value), and FEV1/FVC 65%, suggesting mild obstructive ventilatory dysfunction. However, the diffusing capacity for carbone monoxide (DLco) was 14.3 mL/mm Hg/min (78% of predicted value). Electrocardiograms showed no abnormal findings. Arterial blood gas analysis displayed pH 7.36, Paco2 46 mm Hg, PaO2 73 mm Hg, HCO3 - 25.7 mmol/L, and O2 saturation 94%. Chest radiographs revealed radiation pulmonary fibrosis in both upper lobes and mild thickening in the left pleura (Figure 2). On computer tomography (CT) scans, although there was no local tumor recurrence in the anterior mediastinum, reactive hyperplasia was found in the right para-aortic and paratracheal lymph nodes. In addition, a contrast-enhanced nodule measuring 2.2 cm in the greatest diameter was detected in the posterior segment of the left lower lobe (Figure 3). Positron emission tomography showed a fluorodeoxyglucose (FDG)-absorbing nodule in the left lower lobe pleura (Figure 4). The nodule in the posterior segment of the left lower lobe was resected through video-assisted thoracoscopy, and the surgical specimen showed findings suggestive of type B2 thymoma (Figure 5) and no thymoma cell at the resection margin. The histopathological findings were similar to those obtained 6 years ago. The patient is periodically followed up, and his postoperative course was uneventful without any recurrence of thymoma.
Thymoma and thymic carcinoma are rare neoplasms arising from the epithelial cells of the thymus3,4. However, these tumors represent the most frequently diagnosed tumor of the anterior mediastinum and account for approximately 45% of all of the anterior mediastinal masses4,5. These tumors are reported to occur predominantly in the fifth to sixth decades6. Most thymomas are slow-growing and indolent tumors1. Although 30% to 50% of all thymomas manifest no clinical symptoms at the time of diagnosis7, they are accompanied by dyspnea, chest pain, weight loss, fatigue, cough, superior vena cava syndrome due to compression of and infiltration into the adjacent tissue, hoarseness, dysphagia and pleural/pericardial effusion, which show extremely poor prognoses6. Our patient had no clinical symptoms at the time of diagnosis and predicted a poor prognosis. Prognostic factors for thymoma include metastasis, tumor size (>10 cm), age (≤30 years), lymphocyte predominance, mixed tissue form and paraneoplastic syndromes such as myasthenia gravis, red cell aplasia and hypogammaglobulinemia5,6,8.
By gross examination, thymoma is a lobulated and firm tumor, has tan-pink to gray color and contains cystic spaces, calcification or hemorrhage. In addition, it is encapsulated and invasive and frequently adheres to the surrounding structures9. Our patient had a gray mass that infiltrated into the adjacent connective tissue. Suster and Moran10 described thymomas according to their dominant cell type: predominantly lymphocytic, predominantly epithelial, predominantly mixed, predominantly lymphoepithelial and predominantly spine cell. The World Health Organization (WHO) classified thymomas into 6 types according to the shape of epithelial cells and the proportion of lymphocytes and epithelial cells: types A, B1, B2, B3 and C3. Our patient had a thymoma belonging to type B2 according to the WHO classification system. Type B2 thymoma is characterized by the histologic features of large, polygonal tumor cells that are arranged in a loose network and exhibit large vesicular nuclei with prominent large nucleoli - background population of immature T-cells always present11. Thymoma appears as an ill-demarcated, lobulated mass in the anterior mediastinum on chest radiographs5. Contrast-enhanced chest CT shows a well-demarcated, encapsulated mass, frequently accompanying hemorrhage, necrosis and cyst formation5. In our patient, although the mass was not detected on chest radiographs, chest CT showed a contrast-enhanced nodule in the posterior segment of the left lower lobe with focal thickening. There was no finding of hemorrhage, necrosis or cyst formation.
The main treatment method is complete surgical resection that improves the long-term survival rate12. In previous large-scaled studies, complete resection is an independent prognostic factor by multivariate analysis, and the mortality rate is reported to be 2.5%3. Surgical resection plus postoperative radiation therapy is recommended as a multimodality treatment, and a radiation dose of at least 4,000 cGy is required6. Our patient received radiation therapy with a dose of 5,040 cGy after pleural node excision through video-assisted thoracoscopy. Only a few cases of tumor recurrence after surgical resection of thymoma have been reported1,12-14. In cases with invasive thymoma, the recurrence rate was reported to be about 11% to 36%, whereas encapsulated thymomas recur in approximately 10% of all patients7,12. Recurrence of thymoma has been reported after disease free intervals of more than 10 years13.
Therefore, long-term follow-up is mandatory14. Contrast-enhanced chest CT is the main diagnostic tool and should be performed annually during the follow-up period. The duration of follow-up still remains controversial. Since thymoma recurred 6 years after surgery in our patient and 10 years after surgery in previous reports, it is inferred that patients should be followed up for at least 10 years. Intrathoracic implantation can occur any place in the pleural cavity but occurs most frequently in the mediastinal pleura15. In most cases, local recurrence and distant metastasis are extremely rare14. Since pleural recurrence usually produces no symptoms, it is frequently encountered incidentally during the follow-up period15. Our patient was followed up every 6 months by chest CT. At the time of diagnosis, he had no clinical symptoms, and neither chest CT nor positron emission tomography showed distant metastasis. Intrathoracic recurrence is more often in early stage (I and II) and distant metastases occur often in stages III and IV thymoma13, and 6 years before our patient was diagnosed to Masaoka stage II. In our case, so called 'droplet metastasis', there is no possible way to distinguish between hematogenous metastasis, implantation metastasis during surgical resection and ectopic thymoma. But seeding of tumoral cells during the manipulation of the tumor is possible mechanism, particularly if the mediastinal pleura have been opened15. This kind of problem is more frequent when using minimally invasive technique such as video-assisted thoracoscopy or robotic technology, therefore a long-term follow-up is necessary to exclude the possibility that minimally invasive techniques can expose the patients to a higher risk or pleural relapse15. Though our patient underwent surgical excision by sternotomy, seeding of tumoral cells during the manipulation of the tumor is thought to the possible mechanism. It has generally been recognized that recurrent thymoma can be best treated by surgical resection. Nomori et al.14 reported in a study of 30 patients with recurrent thymoma that prognosis was better in those undergoing complete resection than in those undergoing radiation therapy and that the 5-year survival rate after complete resection of local recurrent thymoma was 100%, whereas that after resection of distant metastatic lesions was 30%.
We reported the case of a 67-year-old patient with invasive thymoma of the anterior mediastinum that showed pleural recurrence 6 years after surgical resection.
Figures and Tables
Figure 1
Chest radiograph shows a well-demarcated, lobulated mass in the left anterior mediastinum (arrow).
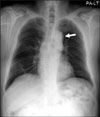
Figure 2
Chest radiograph shows the radiation fibrosis of central areas and mild left pleural thickening but no evidence of newly appearing lesions.
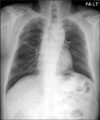
Figure 3
Chest CT scan with a mediastinal window image shows an enhanced pleural nodule (arrow) in the left lower posterior costal pleura.
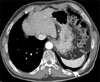
Figure 4
PET CT scan shows slightly increased FDG uptake in the left lower hemithorax (maximal SUV, 1.95) probably due to tear-drop metastasis (arrow). PET CT: positron emission and computer tomography; FDG: fluorodeoxyglucose.
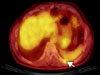
Figure 5
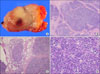
(A) Gross findings of the tumor showing tan-colored nodular pattern with whitish fibrous bands. (B) Microscopic findings showing lobular growth pattern with thick fibrous bands (H&E, ×12.5). (C) Invasion into the surrounding fat tissue (H&E, ×40). (D) High power showing large and polygonal epithelial cells and many number of lymphoid cells. The tumor cells have large nuclei with prominent single nucleoli (H&E, ×200).
References
1. Debnath J, Chawla N, Talwar R, Vohra LS, George RA, Singh HP, et al. Pleural and transdiaphragmatic retroperitoneal metastasis developing two and half years after resection of invasive thymoma. Singapore Med J. 2008. 49:e64–e67.
2. Awad WI, Symmans PJ, Dussek JE. Recurrence of stage I thymoma 32 years after total excision. Ann Thorac Surg. 1998. 66:2106–2108.
3. Gielda BT, Peng R, Coleman JL, Thomas CR, Cameron RB. Treatment of early stage thymic tumors: surgery and radiation therapy. Curr Treat Options Oncol. 2008. 9:259–268.
4. Kondo K. Optimal therapy for thymoma. J Med Invest. 2008. 55:17–28.
5. Kurup A, Loehrer PJ Sr. Thymoma and thymic carcinoma: therapeutic approaches. Clin Lung Cancer. 2004. 6:28–32.
6. Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005. 128:2893–2909.
7. Song HS, Han SB, Kang YW, Jeon YJ, Kim H, Lee SS. Invasive thymoma. Korean J Intern Med. 1987. 33:548–556.
8. Vaideeswar P, Padmanabhan A, Deshpande JR, Pandit SP. Thymoma: a pathological study of 50 cases. J Postgrad Med. 2004. 50:94–97.
9. Welsh JS, Thurman SA, Howard SP. Thymoma and multiple malignancies: a case of five synchronous neoplasms and literature review. Clin Med Res. 2003. 1:227–232.
10. Suster S, Moran CA. Thymoma classification: current status and future trends. Am J Clin Pathol. 2006. 125:542–554.
11. Tomaszek S, Wigle DA, Keshavjee S, Fischer S. Thymomas: review of current clinical practice. Ann Thorac Surg. 2009. 87:1973–1980.
12. Sakada T, Sugio K, Nishioka K, Tsukamoto S, Ushijima C, Yamazaki K, et al. Invasive thymoma with long-term survival by extensive reoperation. Respiration. 1999. 66:167–169.
13. Reddy RH, Shah R, Kumar B, Thorpe JA. Recurrence of stage I thymoma in sternum, 13 years after "complete" excision. Eur J Cardiothorac Surg. 2003. 23:134–135.
14. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Orikasa H, et al. Pulmonary metastasis 12 years after resection of thymoma with microscopic capsule invasion. Jpn J Clin Oncol. 2004. 34:630–633.
15. Lucchi M, Basolo F, Mussi A. Surgical treatment of pleural recurrence from thymoma. Eur J Cardiothorac Surg. 2008. 33:707–711.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download