Abstract
Background
Methods
Results
Figures and Tables
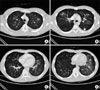 | Figure 1An 18-year-old male patient with pandemic influenza A/H1N1 pneumonia. Chest CT scans obtained in the level I (A), level II (B), level III (C) and level IV (D) showed bilateral and multi-focal ground glass opacities. Left pleural effusion was also found (white arrow). CT: computed tomography. |
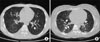 | Figure 2A 27-year-old male (A) and a 42-year-old female (B) with pandemic influenza A/H1N1 pneumonia. Chest CT revealed that multiple ill-defined ground glass opacities in middle to lower lungs with bronchial wall thickening (A, B) (white arrow). Right pleural effusion was also seen (A) (black arrow). CT: computed tomography. |
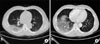 | Figure 3A 55-year-old male patient with pandemic influenza A/H1N1 pneumonia. Chest CT obtained 4 days after onset of symptoms showed a diffuse mixed pattern with ground glass opacities and consolidation in the right lower lobe. CT: computed tomography. |
Table 1

Data are presented as median (IQR) or number (%).
*Score of CURB-65 is an acronym for each of the risk factors measured (confusion, blood urea nitrogen 19 mg/dL or greater, respiratory rate of 30 breaths per minute or greater, systolic blood pressure less than 90 mm Hg, or diastolic blood pressure 60 mm Hg or less, and age 65 or older). Each risk factor scores one point, for a maximum score of 5.
IQR: interquartile range.
Table 2

Values are presented as number (%) unless otherwise indicated.
*Evaluation of bronchial wall thickness and extent of lesion was available in 19 patients, since one had dense consolidation with atelectasis in the left upper lobe, †aortic arch level (level I), tracheal bifurcation level (level II), left inferior pulmonary vein level (level III), and supradiaphragmatic level (2 cm above right diaphragm dome, level IV). Data were presented as number of cases (mean score of extent of lesion), ‡peribronchial artery thickness.
Acknowledgements
Notes
Yee Hyung Kim was the primary investigator and corresponding author who performed collecting and analyzing all the clinical and radiological data. He also made a draft of the manuscript and revised it with interpretation of data; Jun Seong Son was the primary investigator who analyzed the clinical data; Young Kyung Lee reviewed the radiological images of all patients; Cheon Woong Choi, Myung Jae Park, Jee-Hong Yoo, Hong Mo Kang and Jong Hoo Lee were revising the manuscript.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download