Abstract
Background
Hyaluronan (HA) is an unbranched glycosaminoglycan. It has been proposed that HA acts as a vehicle for cytokines due to the strong negative charge on its surface. We hypothesized that HA would function like a cytokine scavenger and reduce the inflammatory signaling cascade and this would lead to improved survival in rats suffering with endotoxemia.
Methods
Endotoxin (Salmonella, 10 mg/kg) or an equal amount of 0.9% NaCl (NS) was injected into the jugular vein of rats. HA (1,600 kDa, 0.35%) or NS was given at 0.1 mL/kg/h for 3 hours. HA or NS infusion was started at 4 hour after endotoxin injection. The rats were divided into the control and HA groups (n=16 for each group). The mean arterial pressure (MAP) was monitored during HA or normal saline infusion. Survival was assessed every 12 hours for 3 days throughout the experiment.
Results
The survival rate (%) of the rats treated with HA was higher (60%) than that of the controls (20%) when HA was infused 4 hours after lipopolysaccharide (LPS) injection. The bronchoalveolar lavage (BAL) fluid of the animals surviving HA or NS infusion 4 hours after LPS showed that the total cell counts and number of neutrophils were significantly (p<0.01) reduced in the HA treated groups compared with that of the controls (total cell count, 9.2×104/mL vs. 61×104/mL; neutrophils, 21×104/mL vs. 0.2×104/mL, respectively). There was no significant MAP difference between the HA or control groups either with or without endotoxin.
Severe, overwhelming infection can result in sepsis that affects the entire body and this can cause fever, a rapid heart rate and other systemic signs. It may often cause lung or kidney damage, and it can ultimately lead to death. When sepsis is associated with severe hypotension or organ damage, the mortality rate reaches 30~40%1. Sepsis is the most common condition requiring admission to the intensive care unit and it is a common cause of acute lung injury2.
In the lung, various types of extracelluar matrix (ECM) such as collagen, elastin and proteoglycans as well as stromal cells are present. The extracellular matrix mediates diverse biological reactions through its interaction with cell surface receptors and this results in stimulated secretion of cytokines and growth factors3. Proteoglycans (PGs) are the basic component of the extracellular matrix, and one or more glycosaminoglycans (GAGs) are attached to its core proteins in the center. The GAGs present in the lung are chondroitin sulfate, dermatan sulfate, heparin sulfate and hyaluronan4.
Hyaluronan is negatively charged, and the structure of GAG is repeated disaccharide units consisting of N-acetylglucosamine and glucuronic acid, and no protein can form a covalent bond with hyaluronan. Generally, hyaluronan with molecular weight greater than 500 kDa is classified as high molecular weight hyaluronan (HMW hyaluronan). HWM hyaluronan is an important component that forms the pulmonary interstitium, and it is in charge of maintaining the compliance of the normal lung and structures5. In contrast, low molecular weight hyaluronan (LMW hyaluronan) with a molecular weight lower than 500 kDa acts as signal transduction mediators in inflammation6,7. HMW hyaluronan mediates anti-inflammatory reactions in diverse diseases. HMW HA treatment has been shown to decrease inflammation in degenerative osteoarthritis8,9 and liver injury10, and to block cigarette-induced emphysema in mice11.
In our study, we examined the effect of the intravenous injection of HMW hyaluronan on the survival rate in a sepsis animal model.
A catheter was inserted to the right jugular vein, and endotoxin (15 mg/kg; S. typhosa ATCC 10749; Sigma, St. Louis, MO, USA) was injected through the jugular vein. 0.9% saline at a volume equivalent to the endotoxin was injected to the control group. In a sepsis rat model, 4 hours after the injection of endotoxin, HMW hyaluronan (0.35% 1,600 kDa; Genzyme, Boston, MA) or the same volume of 0.9% NaCl was infused for 3 hours at a rate of 0.1 mL/kg/h. After the completion of the injection, the animals were transferred to cages, and the survival status of the rats was monitored at 12 hours intervals. If the animals were found to be in distress, ie, unable to eat, ruffled fur or obvious respiratory distress, then they were sacrificed. Bronchoalveolar lavage were performed to examine the changes of inflammatory cells in the alveolus of the surviving rats.
Silastic (0.012 inch I.D, 0.025 inch O.D) catheters were placed in the left carotid artery to monitor systemic artery pressure of the endotoxin injection group and the control group, and the rats in the hyaluronan injection group with or without endotoxemia were placed on a Gould recorder (Model R53400; Glen Burnie, MD, USA) and it recorded at 30 minutes intervals (n=5/group).
The serum cytokines and bronchoalveolar lavage fluid were analyzed prior to the injection of endotoxin, at 4 hours after the injection and 3 hours after the infusion of HMW HA (7 hours after the injection of endotoxin) (n=7/group). The same tests were performed on the rats that survived 72 hours with endotoxemia. The serum was collected and 4 cytokines were measured. Tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) were measured using ELISA kits (R&D Systems, Minneapolis, MN). The lungs were removed en bloc and tubing was inserted into the right main bronchus and secured. The right upper lobe was clamped at the bronchus to prevent the lavage fluid from entering. The right lower lung was lavaged with 2 mL of normal saline 3 times. The neutrophil counts in the bronchoalveolar lavage fluid (BAL) were used to measure the migration of neutrophils into the alveoli, as was previously described12. The total cell counts in the BAL were performed using a hemocytometer. To perform cell differential counts, the cells were fixed on glass slides by use of cytospin and they were stained with geimsa.
The left lungs were inflated with 10% formalin at 20 cm H2O pressure. After at least 48 hours of fixation, the left lung was cut into 0.5 cm thick sections. The sections were embedded in paraffin, cut into 5-µm pieces, mounted on glass slides and stained with hematoxylin-eosin (H-E) for determining the presence and type of lung injury. Pathological scores were obtained based on previous experiments13. Briefly, a blinded reviewer examined 5 fields (2 peripheral and 3 central fields) on each slide for 5 variables related to injury. These variables included a) airway epithelial shedding, b) airway epithelial edema, c) increased cellularity in the airway and parenchymal tissues, d) increased peribronchial and perivascular cuff areas and e) alveolar atelectasis. The total lung injury score was calculated as the sum of each variable semi-quantatively scored as none (0), mild (1), moderate (2), or severe (3).
All the values are presented the mean±standard error of the mean. Analysis of variance was performed for the comparisons among the groups, and the significant cases were analyzed by Scheffe's test. The survival rate of the two groups was compared by the Mantel-Cox log rank test. Blood pressure was compared by two-way repeated measures analysis of variance and a post hoc tests. Statview 4.5 (SAS Institute Inc., Cary, USA) was used for the statistical analysis, and p values lower than 0.05 were considered to be statistically significant.
Hyaluronan (1,600 kDa) was injected through the jugular vein 4 hours after the injection of endotoxin. After 72 hours, 3 of 16 animals (20%) survived in the control group injected with 0.9% NaCl; on the other hand, 9 of 16 animals (60%) survived in the hyaluronan injection group (p<0.04) (Figure 1). Death occurred within 24 hours in most cases, and it was found that the effect of hyaluronan (1,600 kDa) could be observed within 24 hours after injection.
For the histological findings of the lung 3 hours after the infusion of HMW HA or saline in endotoxemic rats, it was observed that in the group injected with saline after the injection of endotoxin, inflammatory cells had infiltrated to the alveoli and overall alveolar collapse was observed. In the hyaluronan treatment group, a relatively lesser infiltration of inflammatory cells to the lung was observed (Figure 2). In regard to the pathological scores, the score was significantly decreased in the hyaluronan injection group (1.1±0.2; p<0.05) as compared to that of the saline injection group (1.9±0.3). It was observed that the increased cells were primarily neutrophils in the bronchoalveolar lavage fluid (Figure 3).
In the control group that received saline, the total number of cells was increased by 20% 7 hours after the injection of endotoxin as compared to the total number of cells at the time point 4 hours after the injection. As compared with the control group, the number of total cells was significantly lower in the hyaluronan treated group at the time point 7 hours after the exdotoxin injection (Figure 3A). On comparing the rats that survived after 72 hours in the 2 groups, there was a trend that the cell count was still higher in the saline injection group than that in the hyaluronan injection group. However, this was not statistically significant. The number of neutrophils also showed a trend similar to the number of all the cells. In the control group, the number of neutrophils in the bronchoalveolar lavage fluid 4 hours and 7 hours after the injection of endotoxin was not significantly different. At the time point 7 hours, in the hyaluronan injection group, the number of neutrophils was significantly reduced to approximately 60% of that of the control group (Figure 3B).
To assess the anti-inflammatory reaction of hyaluronan in the sepsis induced by endotoxin, serum tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) were measured. TNF-α, IL-10 and MIP-2 of both the control group and the hyaluronan injection group showed a trend to be decreased at 7 hours after the injection of endotoxin as compared with that after 4 hours. TNF-α and MIP-2 of the hyaluronan injection group were significantly reduced as compared with that of the control group at 7 hours after the injection of endotoxin, and MIP-2 was decreased by 60% in the hyaluronan treated group as compared with that of the control group (Figure 4).
The arterial blood pressure was measured to examine the effect of the injection of hyaluronan on blood pressure. The injection of endotoxin or hyaluronan did not induce significant changes of the blood pressure as compared with that of saline (n=5 per group, Figure 5).
This study examined whether hyaluronan treatment increases the survival rate in a sepsis animal model prepared by the injection of endotoxin. The mortality rate of the control group that had saline injected after the injection of endotoxin was high at approximately 80% and the survival rate of the hyaluronan treatment group was 40%. A significant difference was observed. A reduction of neutrophils in the bronchoalveolar lavage fluid, a reduction of serum TNF-α and MIP-2 and a reduction of lung tissue damage were observed in the hyaluronan treatment group as compared with that of the control group. The increased survival rate by hyaluronan was found to not be associated with the elevation of blood pressure (Figure 5). The effect of a reduced mortality rate was observed within 24 hours after the development of sepsis in most cases. We think an intravenous injection of hyaluronan it could be an effective treatment method if this is done in the initial stage of the onset of sepsis.
In addition, hyaluronan injection reduced the infiltration of neutrophils to the lung and it decreased the concentration of serum TNF-α and MIP-2. It is suggested that the survival gain of the hyaluronan injection group may be associated with reducing excessive inflammation. Lung inflammation and the serum cytokines were reduced immediately after finishing the 3 hour infusion of hyaluronan, Thus, it is thought that for cases with an excessive inflammatory reaction, such reactions may be effectively reduced in a short time by the administration of hyaluronan.
Neutrophils play an important role in the defense mechanism of our body in the septic condition and they induce excessive inflammatory reactions. Activated neutrophils induce damage to not only bacteria, but also to host tissue. The proteinases released from neutrophils cause damage of adjacent tissue cells14. In the present study, after the administration of hyaluronan, the infiltration of neutrophils to the alveoli was decreased. It is speculated that HMW hyaluronan prevents the infiltration of neutrophils by exerting effects on the surface of inflammatory cells that suppress the release of cytokines, or HMW hyaluronan suppresses additional inflammatory reactions by mediating effects on the neutrophils themselves15,16.
Sepsis may be explained as the excessive synthesis of tumor necrosis factor-α (TNF-α) and other cytokines, as well as a hyper-inflammatory response caused by the abnormal activation of the immune system17. Such excessive release of cytokines may become the cause of multiple organ failure18,19. An increase of the survival rate was not observed when septic patients with increased interleukin-6 were treated with therapy (monoclonal antibody) that suppresses TNF-α20,21. In contrast to most studies that neutralized TNF-α with monoclonal antibody20,21, in our study, the TNF-α was reduced by the intravenous injection of hyaluronan. However, the serum TNF-α was not complete abolished. We think that control of the TNF-α via hyaluronan will be applied to the treatment of septic patients in the future.
When sepsis progresses, the concentration of the inflammation suppressor cytokine IL-10 is elevated22. In our study, IL-10 was elevated in the initial period of sepsis; nonetheless, a difference between the treatment group and the control group was not observed, and similarly, the serum IL-10 at 72 hours was not different between the groups.
In rodents, interleukin-8 similar to that in humans is not detected. The cytokine that is involved in neutrophil migration and chemotaxis in rodents has been shown to be MIP-223,24. In our study, the serum MIP-2 was decreased in the hyaluronan treatment group as compared with that in the control group, and the neutrophils in the bronchoalveolar lavage were decreased in the hyaluronan treatment group as compared with that in the control group. This suggests that hyaluronan reduces the serum concentration of MIP-2 and so it decreases the recruitment of neutrophils to the alveoli.
In conclusion, in our sepsis animal model, excessive inflammatory reactions that developed in the septic condition were suppressed by the injection of hyaluronan and the survival rate was increased.
Figures and Tables
 | Figure 1Intravenous infusion of hyaluronan (LPS+HA) in endotoxemic rats prolonged the animals' survival compared with that of the saline control (LPS+Saline) (n=16 per group). LPS: lipopolysaccharide; HA: hyaluronan. |
 | Figure 2Representative histology findings (hematoxylin and eosin stain, original magnification, ×100). The lungs were removed after three hours of infusion of hyaluroan or 0.9% NaCl in the endotoxemic rats. (A) Control, (B) LPS+0.9% NaCl, (C) LPS+Hyaluronan (1,600 kDa). LPS: lipopolysaccharide. |
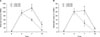 | Figure 3Treatment with hyaluronan (HA) significantly reduced the total number of cells (A) and neutrophils (B) in the bronchoalveolar lavage fluid (n=7 rats per group). *p<0.01 LPS+NS vs. LPS+HA at 7 hours. LPS: lipopolysaccharide; NS: NaCl; HA: hyaluronan. |
 | Figure 4The levels of serum tumor necrosis factor-α (TNF-α) (A), interleukin-10 (IL-10) (B), interleukin-6 (IL-6) (C) and macrophage inflammatory protein-2 (MIP-2) (D) were measured at baseline, 4-, 7- and 72 hour after endotoxemia. Treatment with hyaluronan (HA) significantly reduced the serum levels of TNF-α and MIP at 7 h after endotoxemia compared with that of the control (n=7 rats per group). *p<0.01 LPS+NS vs. LPS+HA at 7 hours. LPS: lipopolysaccharide; NS: NaCl. |
 | Figure 5Effect on the mean arterial pressure (MAP) in rats treated with either hyaluronan (HA) or 0.9% NaCl (NS) one hour after endotoxemia (LPS). Hyaluronan infusion did not cause a significant MAP change in the rats with or without endotoxemia (open triangle=LPS+NS; open circle=LPS+HA; closed triangle=NS; closed circle=HA). |
Acknowledgements
This study was supported by a 2010 grant from The Korean Academy of Tuberculosis and Respiratory Diseases.
References
1. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995. 273:117–123.
2. Irish Critical Care Trials Group. Acute lung injury and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care. 2008. 12:R30.
3. Starcher BC. Lung elastin and matrix. Chest. 2000. 117:5 Suppl 1. 229S–234S.
4. Hance AJ, Crystal RG. The connective tissue of lung. Am Rev Respir Dis. 1975. 112:657–711.
5. Cantor JO, Cerreta JM, Keller S, Turino GM. Modulation of airspace enlargement in elastase-induced emphysema by intratracheal instillment of hyaluronidase and hyaluronic acid. Exp Lung Res. 1995. 21:423–436.
6. McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996. 98:2403–2413.
7. Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005. 11:1173–1179.
8. Brandt KD, Smith GN Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000. 43:1192–1203.
9. Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998. 61:125–135.
10. Nakamura K, Yokohama S, Yoneda M, Okamoto S, Tamaki Y, Ito T, et al. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J Gastroenterol. 2004. 39:346–354.
11. Cantor JO, Cerreta JM, Ochoa M, Ma S, Chow T, Grunig G, et al. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp Lung Res. 2005. 31:417–430.
12. Quinn DA, Moufarrej R, Volokhov A, Syrkina O, Hales CA. Combined smoke inhalation and scald burn in the rat. J Burn Care Rehabil. 2003. 24:208–216.
13. Choi WI, Kwon KY, Kim JM, Quinn DA, Hales CA, Seo JW. Atelectasis induced by thoracotomy causes lung injury during mechanical ventilation in endotoxemic rats. J Korean Med Sci. 2008. 23:406–413.
14. Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005. 36:956–962.
15. Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005. 26:637–643.
16. Alam CA, Seed MP, Freemantle C, Brown J, Perretti M, Carrier M, et al. The inhibition of neutrophil-endothelial cell adhesion by hyaluronan independent of CD44. Inflammopharmacology. 2005. 12:535–550.
17. Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007. 170:1435–1444.
18. Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999. 27:1309–1318.
19. van der Poll T, Lowry SF. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995. 3:1–12.
20. Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med. 2001. 29:765–769.
21. Macias WL, Nelson DR, Williams M, Garg R, Janes J, Sashegyi A. Lack of evidence for qualitative treatment by disease severity interactions in clinical studies of severe sepsis. Crit Care. 2005. 9:R607–R622.
22. Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med. 2005. 33:12 Suppl. S468–S471.
23. Schmal H, Shanley TP, Jones ML, Friedl HP, Ward PA. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996. 156:1963–1972.
24. Standiford TJ, Kunkel SL, Greenberger MJ, Laichalk LL, Strieter RM. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996. 59:24–28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download