Abstract
Pulmonary capillary hemangiomatosis (PCH) is a rare disease of unknown etiology that is characterized by nodules composed of infiltrating capillary blood vessels. Herein, we describe a case of a PCH-like lesion that was detected by chest computed tomography. Transthoracic needle aspiration resulted in life-threatening hemorrhage. The patient was followed for seven years. He remained in good health and a follow up image showed little interval change.
Pulmonary capillary hemangiomatosis (PCH) has been described as multiple nodules in the lung parenchyma or bronchovascular walls composed of infiltrating thin-walled capillary blood vessels1. The typical described clinical course for patients with PCH is that of unrelenting symptoms of pulmonary hypertension and eventual death2. Unfortunately, a definitive diagnosis of PCH usually is not made until autopsy1. Here we describe a case of PCH-like lesion that was detected by chest computed tomography (CT) and followed for seven years.
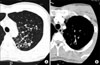
A 17-year-old man was referred to Seoul Adventist Hospital in January 2003 for detailed evaluation of abnormal lesion that had been discovered by chest X-ray and CT. Chest high resolution CT (HRCT) scan on admission showed ill-defined hazy densities and interlobular and intralobular septal thickenings in left upper lobe (LUL) (Figure 1). His physical condition was good, and there were no signs of dyspnea or hemoptysis. On admission, laboratory findings were normal. He had been in relatively good health until admission except mild exertional dyspnea.
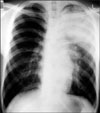
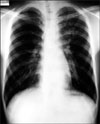
On the first hospital day, bronchoscopy was performed. There was no endobronchial lesion in the exam. Bronchial washing was done in LUL bronchus and the result for acid fast bacilli stain was negative. On the second hospital day, chest magnetic resonance image (MRI) was performed. MRI showed reticular shaped iso-signal intensity on T1 weighted image (WI) and mottled reticular shaped high signal intensity on T2WI (Figure 2). Dysplasia of branching vessel originating from thoracic aorta was noted. On the third hospital day, CT guided transthoracic needle aspiration (TTNA) was performed (Figure 3). After the biopsy, cough and hemoptysis with dyspnea were developed. Chest X-ray showed ill defined hazy density with focal air bronchogram in LUL (Figure 4). Follow up CT was performed and newly visible ill-defined air space consolidation in LUL was noted (Figure 5). Laboratory examination showed the decrease level of hemoglobin (11.5 g/dL) and the patient was transferred to the intensive care unit. On the ninth hospital day, hemoptysis, cough, and dyspnea were subsided. Because of small amount of specimen, biopsy result showed only a portion of fibrous tissue. On the 15th hospital day, X-ray showed improvement of consolidation in LUL (Figure 6). The patient was discharged. Follow up X-ray showed further improvement of consolidation (Figure 7).
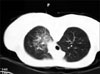
The patients were regularly followed up, and no significant problem was noted. In 2008, he joined the army and followed for two years at Armed Forces Yangju Hospital. During the period of service, he was in relatively good health except intermittent small amount of blood tinged sputum and mild exertional dyspnea. In 2010, enhanced chest CT was followed up for the routine check (Figure 8). CT image showed little interval change compared with previous one taken in 2003. Interlobular septal thickening was still noted with little change. Enhanced vascular structure was also noted inside thickened interlobular septum. He finished service in May 2010 with good health status.
PCH is a rare disorder characterized by proliferating capillaries that invade the pulmonary interstitium and alveolar septae, and occlude the pulmonary vasculature3. Age at onset may vary between 6 and 71 years old, but the majority of patients are between 20 and 40 years old. The earliest symptom is a gradual onset of shortness of breath on exertion4. Clinical signs and symptoms include progressive pulmonary hypertension with dyspnea, cough, fatigue, and weight loss. Hemoptysis occurs in more than 30% of cases5.
Although the diagnosis of PCH often occurs postmortem, one useful diagnostic tool is HRCT. The most characteristic findings on HRCT in patients with PCH were diffuse thickening of the interlobular septae and small, centrilobular, poorly circumscribed nodular opacities6-11. Dufour et al.6 showed that these features correlated well with pathologic abnormalities. Pathologic examination of samples collected from the patients with PCH showed diffuse nodular proliferation of small capillary-sized vessels that widened the interalveolar septae with formation of small nodules or sheets of back-to-back capillary vessels. Eltorky et al.11 also showed that interlobular septal thickening and centrilobular nodularity in HRCT correlated with the focal angiomatoid proliferation within the interlobular interstitium and the venous and alveolar walls, respectively. These imaging findings above were well compatible with our case. Although exact pathologic diagnosis was not made in our case, septal thickening in HRCT probably reflect underlying nodular proliferation of small vessel. Additionally, enhance image of MRI and CT clearly showed that the lesion was composed of small vessel and TTNA resulted in massive hemorrhage in lung. These findings strongly favor the diagnosis of PCH.
The diagnosis of PCH is difficult and requires a high degree of clinical suspicion and accurate pathologic study. Multiple sampling in pulmonary biopsy is essential because of the patchy nature of the disease12. Almagro et al.13 reviewed 37 cases of PCH. Among them, diagnosis was made with pulmonary biopsy in only 3 cases. The rest were wrongly classified as pulmonary fibrosis, pulmonary veno-occlusive disease, hemosiderosis, and other entities, and definitive diagnosis was reached only after lung transplantation or autopsy. Also, in our case, the specimen of TTNA showed only a portion of fibrous tissue. The lesson from our case is that when suspicious of PCH, biopsy procedure such as TTNA or transbronchial lung biopsy should be avoided. This procedure is not only inadequate for the diagnosis but also very dangerous.
PCH is a rapidly progressive disease, with a median survival of 3 years from the time of diagnosis. According to the review by al-Fawaz et al.14, the condition usually progresses rapidly, causing death from pulmonary hypertension and/or bleeding without effective treatment. Of the 19 cases, four underwent lung transplantation, one had pneumonectomy, and one was treated with interferon α-2a. These treatments reportedly resulted in improvements in their conditions. However, the other 13 cases died within a total clinical course ranging from several months to several years, and all showed progression of dyspnea, exercise intolerance, cardiac failure, and/or hemoptysis during this period.
In contrast to the active and progressive nature of the disease in above cases, our case has been followed up for seven years during which there has been no clinical deterioration or aggravation of the imaging findings. Although usually the prognosis of PCH is poor and median survival is short, there was a report of stable case of PCH. Takiguchi et al.15 described a case of 29 year old man presented with hemoptysis. The diagnosis of PCH was established by typical HRCT findings and open lung biopsy. The patient was followed for 3.5 years and had no symptoms except for an occasional small amount of hemoptysis. His exercise capacity was good and had no decrease in oxygen saturation. HRCT scans performed 40 months after diagnosis showed little interval change with no increase in the number or size of the lesion. The clinical course of above case was well compatible with ours.
Here we describe a case of PCH-like lesion that was followed for seven years. When the image finding was suspicious of PCH and the symptom of patient was mild, follow up and observation can be an option rather than biopsy. Further studied about the patient with PCH is needed.
Figures and Tables
Figure 1
HRCT showed ill-defined nodular densities and interlobular and intralobular septal thickenings in LUL. HRCT: high resolution computed tomography.
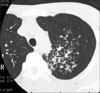
Figure 2
MRI showed reticular shaped iso-signal intensity of T1WI (A) and mottled reticular shaped high signal intensity on T2WI (B). Dysplasia of branching vessel originating from thoracic aorta was also noted on T2WI (C). MRI: magnetic resonance image; WI: weighted image.
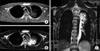
Figure 4
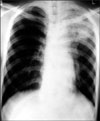
Chest X-ray after TTNA showed newly developed hazy density in LUL. TTNA: transthoracic needle aspiration; LUL: left upper lobe.
References
1. Tron V, Magee F, Wright JL, Colby T, Churg A. Pulmonary capillary hemangiomatosis. Hum Pathol. 1986. 17:1144–1150.
2. Fernández-Alonso J, Zulueta T, Reyes-Ramirez JR, Castillo-Palma MJ, Sanchez-Román J. Pulmonary capillary hemangiomatosis as cause of pulmonary hypertension in a young woman with systemic lupus erythematosus. J Rheumatol. 1999. 26:231–233.
3. Case records of the Massachusetts General Hospital Weekly clinicopathological exercises. Case 38-2000. A 45-year-old woman with exertional dyspnea, hemoptysis, and pulmonary nodulas. N Engl J Med. 2000. 343:1788–1796.
4. Lippert JL, White CS, Cameron EW, Sun CC, Liang X, Rubin LJ. Pulmonary capillary hemangiomatosis: radiographic appearance. J Thorac Imaging. 1998. 13:49–51.
5. de Perrot M, Waddell TK, Chamberlain D, Hutcheon M, Keshavjee S. De novo pulmonary capillary hemangiomatosis occurring rapidly after bilateral lung transplantation. J Heart Lung Transplant. 2003. 22:698–700.
6. Dufour B, Maître S, Humbert M, Capron F, Simonneau G, Musset D. High-resolution CT of the chest in four patients with pulmonary capillary hemangiomatosis or pulmonary venoocclusive disease. AJR Am J Roentgenol. 1998. 171:1321–1324.
7. Hansell DM. Small-vessel diseases of the lung: CT-pathologic correlates. Radiology. 2002. 225:639–653.
8. El-Gabaly M, Farver CF, Budev MA, Mohammed TL. Pulmonary capillary hemangiomatosis imaging findings and literature update. J Comput Assist Tomogr. 2007. 31:608–610.
9. Kothari SS, Jagia P, Gupta A, Singh N, Ray R. Images in cardiovascular medicine. Pulmonary capillary hemangiomatosis. Circulation. 2009. 120:352–354.
10. Lawler LP, Askin FB. Pulmonary capillary hemangiomatosis: multidetector row CT findings and clinico-pathologic correlation. J Thorac Imaging. 2005. 20:61–63.
11. Eltorky MA, Headley AS, Winer-Muram H, Garrett HE Jr, Griffin JP. Pulmonary capillary hemangiomatosis: a clinicopathologic review. Ann Thorac Surg. 1994. 57:772–776.
12. Ishii H, Iwabuchi K, Kameya T, Koshino H. Pulmonary capillary haemangiomatosis. Histopathology. 1996. 29:275–278.
13. Almagro P, Julià J, Sanjaume M, González G, Casalots J, Heredia JL, et al. Pulmonary capillary hemangiomatosis associated with primary pulmonary hypertension: report of 2 new cases and review of 35 cases from the literature. Medicine (Baltimore). 2002. 81:417–424.
14. al-Fawaz IM, al Mobaireek KF, al-Suhaibani M, Ashour M. Pulmonary capillary hemangiomatosis: a case report and review of the literature. Pediatr Pulmonol. 1995. 19:243–248.
15. Takiguchi Y, Uruma T, Hiroshima K, Motoori K, Watanabe R, Hamaoka T, et al. Stable pulmonary capillary haemangiomatosis without symptomatic pulmonary hypertension. Thorax. 2001. 56:815–817.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download