Abstract
Recently, a novel influenza A (H1N1) has been recognized as the cause of a worldwide respiratory infection outbreak. Although the symptoms of a novel influenza A (H1N1) are usually mild, the disease can cause severe illness and death. A complication of novel influenza A (H1N1) is pneumomediastinum, a rarely reported condition. We report a case of influenza A (H1N1) complicating pneumomediastinum with subcutaneous emphysema, which had initially presented with blood tinged sputum and chest pain. In addition, we demonstrate bronchoalveolar lavage in influenza A (H1N1).
A novel influenza A (H1N1) has been known as the cause of a worldwide respiratory infection outbreak. A novel influenza A (H1N1) usually causes febrile respiratory symptoms, but severe illness and death in previously healthy persons has been reportd1,2. The complicating pneumomediastinum of this have only rarely been reported. The finding of bronchoalvolar lavage is unknown. We experience a case of influenza A (H1N1) complicating pneumomediastium and perform bronchoalveolar lavage.
A previously healthy 18-year-old high school student was admitted to the emergency room of this hospital. On day 1 before admission he developed a sore throat and febrile sense. On admission day, his chief complaint was chest pain and blood tinged sputum associated with cough, sputum, fever, chills and dyspnea. There was neither vomiting nor a history of trauma. The patient had no history of smoking, alcohol drinking or drug use, nor did he have a remarkable personal or familial history. There was no history of recent travel. He was living at apartment in urban area.
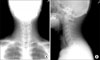
On arrival at hospital, his physical examination revealed the following results: heart rate 90 beats/min, temperature 39.0℃, respiratory rate 20 breaths/min, and blood pressure 120/70 mm Hg. Oxygen was supplied by nasal canula. He had an acutely ill looking appearance. There was subcutenous emphysema in both sides of the neck. A chest examination revealed coarse breathing sounds and rales without wheezing. A cardiac examination revealed a regular heart beat. CBC count showed the following: Hb 16.9 g/dL, hematocrit 47.8%, WBC 12,000/mm-3, segment neutrophil 88.3% and platelet 177,000/µL. hsCRP was increased to 7.76 mg/dL and ESR was normal. Arterial blood gas analysis had a pH of 7.439, a PCO2 of 32.3 mm Hg, PO2 of 102.3 mm Hg, HCO3 22.1 meq/L and Saturation 98.2% by a nasal canula 2 L oxygen supplement. Biochemical data was not significant except AST 54 IU/L, CPK 975 IU/L. Culture of sputum, blood and urine did not show any bacterial pathogen; tuberculosis was also ruled out. Urine Streptococcus pneumonia Ag, Legionella Ag was negative. Real time PCR to detect influenza A (H1N1) was positive. We performed real time-PCR by throat swab specimen. There was no evidence of AIDS, VDRL. On bronchial alveolar lavage, Real time-PCR of influenza A (H1N1) was positive. There was no evidence of influenza B, RSV A, B, parainfleuenza virus, Corona virus 229E/NL63, OC43/HKU1, Rhino virus A/B, Adenovirus and Metapneumovirus. Also we found negative results of TB-PCR and CMV PCR. Cell count of brochial alveolar lavage was Lymphocytes 42%, eosinophils 3%, neutrophils 1% and macrophages 54%. On lymphocyte subset, CD4/CD8 was decreased (0.37). There was no endobronchial lesion with little secretion at bronchoscopic finding. There was obtained boody fluid but no alveolar hemorrhage was found at bronchial alveolar lavage. ECG showed normal sinus rhythm. Chest X-ray showed peribronchial haziness in both lungs (Figure 1). Neck AP and lateral showed subcutaneous emphysema in neck and upper thorax (Figure 2). HRCT showed pneumomediastinum, subcutaneous inter- and intra-muscular emphysema in scan covered lower neck and multiple peribronchial ground grass in both lungs (Figure 3).
He required oxygen supplementation (2 L/min) and was treated with oseltamivir 75 mg bid for 5 days. Three days after in admission he was fever-free and he felt symptomatically improved. We found, on Chest X-ray, that the peribronchial haziness in both lungs had progressively improved. On days 7 of hospitalization, bronchoscopy was performed because he still had blood tinged sputum. There was no active bleeding focus or blood clots in bronchus. He was discharged from the hospital on day 10. After 1weeks, follow up HRCT showed that pneumomediastinum and subcutaneous emphysema had disappeared, and peribronchial ground grass opacity was improved (Figure 4).
Recently, a Novel Swine-Origin influenza A (H1N1) virus was identified in humans in Mexico and the United States. Its worldwide spread was rapid; influenza A (H1N1) virus was genetically and antigenically unrelated to human seasonal influenza viruses and genetically related to virus circulating in swine1-3. It has been reported to be a triple reassortant influenza virus from swine, avian and human. The Spanish flu pandemic virus was relatively mild first but showed more virulence when it returned in the winter. There are concerns that this virus may mutate or reassort with existing influenza viruses, giving rise to more transmissible or more pathogenic viruses1. Most patients had mild symptoms but some required hospital care. These more severe cases usually had pneumonia and dehydration. Certain hospitalized cases have included rapidly progressive lower respiratory tract infections ranging from self-limited to respiratory failure and death3. Influenza A (H1N1) can cause severe acute repiratory distress syndrome and death in previously healthy persons who are young to middle aged3. Half of all patients who died were between 13 and 47 years of age. Others reported as younger than 19 years of age was 43%2.
Spontaneous pneumomediastinum (SPM) is uncommon. Usually Its occurrence is correlated with status asthmaticus with bronchitis4,5. Pneumomediastinum is identified as the presence of air in the mediastinum. A biomodal peak is shown in children younger than 7 years and in youth, aged 13 to 17 years4. It is usually caused by respiratory infection or inflammation and leads to intrathoracic pressure increases followed by rupture of a pulmonary alveolus surrounding bronchioles and pulmonary vessels5. The air extends into the mediastinum by the hilum along the peribronchovascular sheaths. Excess pressure can be caused by valsalva manuvers like coughing and vomiting, and conditions like as asthma, cystic fibrosis, and bronchitis. Up till now there have been no reported cases of pneumomediastinum complicating pneumonia in adolescents by a novel swine origin influenza A (H1N1). Only Hasegawa et al.6 has reported 2 patients who have had asthma or asthmatic symptoms. Our patient, though, showed unusual clinical signs such as blood tinged sputum and no wheezing, radiologic signs that are typical of pneumomediastinum with pneumonia.
Yun et al.7 described clinical characteristics and radiologic features of chest X-ray and HRCT of 18 patients with influenza A (H1N1). Patients had fever with or without accompanying symptoms of cough, sputum, nasal symptoms, myalgia and gastrointestinal symptoms such as diarrhea and vomiting. Combined with ground grass opacities and nodules this may be associated with influenza A (H1N1)7. The ground grass opacities were similar in our case except pneumomediastinum. Choi et al.8 reported 17 patients, one of whom was admitted with severe hypoxemia but died despite intensive resuscitative treatments. Most of the patients had multiple consolidations and infiltratons. One patient had pleural effusion with consolidation. Usually influenza A (H1N1), in contrast, causes mild symptom and good prognosis. To our knowledge, we are the first to describe bronchoalveolar lavage findings associated with an influenza A (H1N1) virus infection. In normal bronchoalveolar lavage there are about 85~95% alveolar macrophages, about 7~12% lymphocytes, about 1~2% neutrophils and less than 1% eosinophils9. Usually in viral pneumonia, about 30% of the cell are lymphocytes, about 10% are neutrophils and about 50~60% are alveolar macrophages10. In our case of bronchoalveolar lavage, the number alveolar macrophages are similar to that of a usual viral infection (54%), but the lymphocytes is mildly higher (42%), as are the eosinophils (3%), and neutrophils are lower (1%). This information will be helpful to many clinician regarding characteristics of influenza A (H1N1) virus infection.
Figures and Tables
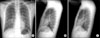 | Figure 1Chest radiograph on admission showed pneumomediastinum and peribronchial haziness in both lungs (A~C). |
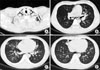 | Figure 3High resolution computed tomography of chest on admission. (A) Showed subcutaneous inter-, intra-muscular emphysema in scan covered lower neck. (B~D) Peribronchial ground grass opacity in both lower lobe, right middle lobe, superior and inferior lingular segment of left upper lobe, posterior segment of right upper lobe. |
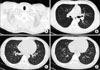 | Figure 4High resolution computed tomography of chest after 2 weeks. (A) Disappeared subcutaneous inter-, intra-muscular emphysema in scan covered lower neck. (B~D) Disappeared pneumomediastinum and improved peribronchial ground grass opacity in both lower lobe, right middle lobe, superior and inferior lingular segment of left upper lobe, posterior segment of right upper lobe. |
References
1. Schnitzler SU, Schnitzler P. An update on swine-origin influenza virus A/H1N1: a review. Virus Genes. 2009. 39:279–292.
2. Centers for Disease Control and Prevention (CDC). Hospitalized patients with novel influenza A (H1N1) virus infection - California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:536–541.
3. ANZIC Influenza Investigators. Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009. 361:1925–1934.
4. Bullaro FM, Bartoletti SC. Spontaneous pneumomediastinum in children: a literature review. Pediatr Emerg Care. 2007. 23:28–30.
5. Newcomb AE, Clarke CP. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest. 2005. 128:3298–3302.
6. Hasegawa M, Hashimoto K, Morozumi M, Ubukata K, Takahashi T, Inamo Y. Spontaneous pneumomediastinum complicating pneumonia in children infected with the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. 2010. 16:195–199.
7. Yun TJ, Kwon GJ, Oh MK, Woo SK, Park SH, Choi SH, et al. Radiological and Clinical Characteristics of a Military Outbreak of Pandemic H1N1 2009 Influenza Virus Infection. Korean J Radiol. 2010. 11:417–424.
8. Choi WJ, Kim WY, Kim SH, Oh BJ, Kim W, Lim KS, et al. Clinical characteristics of pneumonia in hospitalized patients with novel influenza A (H1N1) in Korea. Scand J Infect Dis. 2010. 42:311–314.
9. Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987. 135:250–263.
10. Magnan A, Mege JL, Reynaud M, Thomas P, Capo C, Garbe L, et al. Monitoring of alveolar macrophage production of tumor necrosis factor-alpha and interleukin-6 in lung transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1994. 150:684–689.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download