Abstract
Background
Transglutaminase-2 (TG-2) has been reported to play an important role in the process of fibrosis. However, TG-2 studies on fibroproliferation of acute lung injury (ALI) are absent. The purpose of this study was to investigate the role of TG-2 in the fibroproliferation of lipopolysaccharide (LPS)-induced ALI.
Methods
The male C57BL/6 mice of 5 weeks age were divided into 3 groups; control group (n=30) in which 50 µL of saline was given intratracheally (IT), LPS group (n=30) in which LPS 0.5 mg/kg/50 µL of saline was given IT, and LPS+Cyst group treated with intraperitoneal 200 mg/kg of cystamine, competitive inhibitor of TG-2, after induction of ALI by LPS. TG-2 activity and nuclear factor (NF)-κB were measured in lung tissue homogenate. Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, myeloperoxidase (MPO), and transforming growth factor (TGF)-β1 were measured using bronchoalveolar lavage fluids. Histopathologic ALI score and Mallory's phosphotunistic acid hematoxylin (PTAH) for collagen and fibronectin deposition were performed.
Results
The TG-2 activities in the LPS group were significantly higher than the control and LPS+Cyst groups (p<0.05). The TNF-α and IL-1β concentrations and NF-κB activity were lower in the LPS+Cyst group than the LPS group (p<0.05). The LPS+Cyst group showed lower MPO, ALI score, TGF-β1 concentration, and Mallory's PTAH stain than the LPS group, but the differences were not significant (p>0.05).
Figures and Tables
Figure 1
(A) The time course of transglutaminase (TG)-2 activity. Thirty six hours after intratracheal instillation of lipopolysaccharide (LPS, 0.5 mg/kg in 50 µL of saline), TG-2 activity showed significantly high activity compared with the control, 3, 6, 12, and 24 hours (*p<0.05). At 48 hours and 72 hours, TG-2 activities decreased, but not significant compared with the activity at 36 hours (†p>0.05). (B) The response of TG-2 activity for LPS doses. Instillation of LPS 1.0 mg/kg in 50 µL of saline showed an increased trend of TG-2 activity, but not significant compared with the activity of LPS 0.5 mg/kg (‡p=0.248). (C) The dose response of TG-2 activity for cystamine. At the cystamine dose of 200 mg/kg, TG-2 activity decreased significantly compared with the LPS group (§p=0.009), which was not different with the control group (∥p=0.806).

Figure 2
The time course of acute lung injury (ALI) parameters. The concentrations of tumor necrosis factor (TNF)-α (A), interleukin (IL)-1β (B), and IL-6 (C) in bronchoalveolar lavage fluid (BALF) and nuclear factor (NF)-κB activity (G) in lung tissue homogenates showed peaks at 6 hours after lipopolysaccharide (LPS) administration. Transforming growth factor (TGF)-β1 (D) and myeloperoxidase (MPO) activity (E) were highest at 36 hours, and ALI score (F) showed peak at 48 hours (*p<0.05, compared with the control and other time points).

Figure 3
Overall study design. Control: control group; LPS: lipopolysaccharide group; LPS+Cyst: LPS+cystamine group; BAL: bronchoalveolar lavage; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; IL-6, interleunkin-6; BALF: bronchoalveolar lavage fluid; NF-κB: nuclear factor-κB; TGF-β1: transforming growth factor-β1; Mallory's PTAH: Mallory's phosphotunstic acid hematoxylin; ALI: acute lung injury.

Figure 4
Transglutaminase (TG)-2 activity (A), tumor necrosis factor (TNF)-α (B), and interleukin (IL)-1β (C) were significantly decreased in the lipopolysaccharide (LPS)+Cyst group compared with the LPS group (*p<0.05). IL-6 (D) concentration was higher than the LPS group, but not significant (†p>0.05).

Figure 5
Nuclear factor (NF)-κB activity (A) was significantly decrease in the lipopolysaccharide (LPS)+Cyst group compared with the LPS group (*p=0.028). Myeloperoxidase (MPO) activity (B) and transforming growth factor (TGF)-β1 concentration (C) in the LPS+Cyst group was lower than the LPS group, but not significant (†p>0.05).

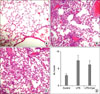
Figure 6
Histopathologic examination shows significantly higher levels of intra-alveolar exudates, inflammatory infiltration, hemorrhage, and interstitial edema in the lipopolysaccharide (LPS) group (B), compared with the control group (A). The LPS+Cyst group (C) showed similar findings with the LPS group. Acute lung injury (ALI) score (D) was not different between the LPS and LPS+Cyst groups (*p=0.171) (H&E stain, ×100).
References
1. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005. 353:1685–1693.
2. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.
3. Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999. 210:29–35.
4. McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994. 150:90–94.
5. Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999. 281:354–360.
6. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003. 348:683–693.
7. Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, et al. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999. 160:1910–1915.
8. Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000. 162:1783–1788.
9. Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002. 368:377–396.
10. Folk JE, Finlayson JS. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977. 31:1–133.
11. Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int. 2002. 40:85–103.
12. Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000. 41:1–27.
13. Johnson TS, Skill NJ, El Nahas AM, Oldroyd SD, Thomas GL, Douthwaite JA, et al. Transglutaminase transcription and antigen translocation in experimental renal scarring. J Am Soc Nephrol. 1999. 10:2146–2157.
14. Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol. 2008. 173:631–642.
15. Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, et al. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am J Physiol. 1997. 272:G281–G288.
16. Johnson TS, Fisher M, Haylor JL, Hau Z, Skill NJ, Jones R, et al. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J Am Soc Nephrol. 2007. 18:3078–3088.
17. Qiu JF, Zhang ZQ, Chen W, Wu ZY. Cystamine ameliorates liver fibrosis induced by carbon tetrachloride via inhibition of tissue transglutaminase. World J Gastroenterol. 2007. 13:4328–4332.
18. Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003. 116:217–224.
19. Shin DM, Jeon JH, Kim CW, Cho SY, Lee HJ, Jang GY, et al. TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008. 22:2498–2507.
20. Verderio EA, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids. 2004. 26:387–404.
21. Kim JH, Suk MH, Yoon DW, Kim HY, Jung KH, Kang EH, et al. Inflammatory and transcriptional roles of poly (ADP-ribose) polymerase in ventilator-induced lung injury. Crit Care. 2008. 12:R108.
22. Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005. 288:L333–L341.
23. Jeitner TM, Delikatny EJ, Ahlqvist J, Capper H, Cooper AJ. Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem Pharmacol. 2005. 69:961–970.
24. Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000. 21:435–466.
25. Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997. 156:840–845.
26. Steinberg KP, Hudson LD. Acute lung injury and acute respiratory distress syndrome: the clinical syndrome. Clin Chest Med. 2000. 21:401–417.
27. Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome: a study in mechanically ventilated patients. Chest. 1995. 107:196–200.
28. Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003. 31:4 Suppl. S258–S264.
29. Grenard P, Bates MK, Aeschlimann D. Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem. 2001. 276:33066–33078.
30. Park KC, Chung KC, Kim YS, Lee J, Joh TH, Kim SY. Transglutaminase 2 induces nitric oxide synthesis in BV-2 microglia. Biochem Biophys Res Commun. 2004. 323:1055–1062.
31. Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, Ha KS. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun. 2003. 305:633–640.
32. Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, et al. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem. 2004. 279:15032–15039.
33. Lorand L, Weissmann LB, Epel DL, Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976. 73:4479–4481.
34. Scott KF, Meyskens FL Jr, Russell DH. Retinoids increase transglutaminase activity and inhibit ornithine decarboxylase activity in Chinese hamster ovary cells and in melanoma cells stimulated to differentiate. Proc Natl Acad Sci U S A. 1982. 79:4093–4097.
35. Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, et al. Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol. 2003. 98:1813–1820.
36. Kuncio GS, Tsyganskaya M, Zhu J, Liu SL, Nagy L, Thomazy V, et al. TNF-alpha modulates expression of the tissue transglutaminase gene in liver cells. Am J Physiol. 1998. 274:G240–G245.
37. Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, et al. Induction of pathogenic sets of genes in macrophages and neurons in Neuro-AIDS. Am J Pathol. 2003. 162:2041–2057.
38. Lee J, Kim YS, Choi DH, Bang MS, Han TR, Joh TH, et al. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004. 279:53725–53735.
39. Griffin M, Smith LL, Wynne J. Changes in transglutaminase activity in an experimental model of pulmonary fibrosis induced by paraquat. Br J Exp Pathol. 1979. 60:653–661.
40. Suh GY, Ham HS, Lee SH, Choi JC, Koh WJ, Kim SY, et al. A Peptide with anti-transglutaminase activity decreases lipopolysaccharide-induced lung inflammation in mice. Exp Lung Res. 2006. 32:43–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download