Abstract
We report the case of a 68-year-old man with a stromal tumor of uncertain malignant potential (STUMP), which had metastasized to the lung. The patient complained of an enlarged mass in the anterior chest. Chest computed tomography (CT) showed a sternal abscess with multiple nodules in both lungs. A thoracoscopic lung biopsy of the nodules and incision/drainage of the sternal mass were performed simultaneously. CT of the pelvis revealed an enlarged prostate with irregular cystic lesions in the pelvis. Prostate biopsy was done and demonstrated hypercellular stroma with minimal cytological atypia, a distinct pattern of STUMP. The sternal abscess proved to be tuberculosis and the lung lesion was consistent with STUMP, which had spread from the prostate. However, to our knowledge, the tuberculous abscess might not be assoicated with STUMP in the lung. The patient refused surgical prostatectomy and was discharged with anti-tuberculosis medication. On one-year follow up, the patient had no evidence of disease progression.
Prostatic stromal tumors are distinct and rare lesions originating from specialized hormonally responsive mesenchymal cells of the prostate. Gaudin et al.1 grouped these tumors into two categories: prostatic stromal tumor of uncertain malignant potential (STUMP) and prostatic stromal sarcomas (PSS), based on the degree of hypercellularity, the presence of mitotic figures, necrosis, and stromal overgrowth. The neoplastic nature of prostatic STUMP remains controversial as the incidence is relatively rare and results of long term follow-up have not been established. However, there have been a few reports of high recurrence, spreading to contiguous organs, and metastasis1-8. We describe a case of prostatic STUMP, presenting with multiple lung metastasis found incidentally on chest X-ray.
A 68-year-old man admitted to the hospital with complaints of a palpable mass in the sternal area. He had recognized the palpable mass one month prior to admission and a history of hypertension for one year.
His vital signs were stable. Chest examination revealed a thumb-sized palpable tender mass in the anterior chest. Digital rectal examination revealed an enlarged prostate with a smooth border. Initial laboratory findings were unremarkable except serum prostate specific antigen (PSA), 5.86 ng/mL (normal, <5.0 ng/mL). Initial chest X-ray revealed small multiple nodules in both lung fields. Chest computed tomography (CT) revealed a sternal abscess and multiple round nodules scattered in both lung fields, suggesting lung metastasis or septic embolization (Figure 1). Percutaneous needle aspiration was performed on the lung nodules but the specimen was inadequate. Abdomen pelvic CT showed an enlarged prostate with irregular cystic lesions and a 2 cm sized nodular enhancing mass in the right seminal vesicle (Figure 2). Cystoscopy revealed mild prostatic enlargement without a definite mass lesion in the bladder. Video assisted thoracoscopic surgery for lung biopsy was performed. Incision and drainage of the parasternal abscess was done simultaneously. Regarding the abscess, Acid fast bacillus (AFB) stain/PCR for M. tuberculosis showed positive results and histology was compatible with tuberculosis. We started anti-tuberculous medication. Prostatic lesion via needle aspiration biopsy confirmed hypercellular stromal cells with no cytological atypia and absence of mitosis or necrosis, demonstrating prostatic STUMP (Figure 3A). On immunohistochemical staining, the tumor displayed positive for progesterone receptor, estrogen receptor, and desmin, and negative for CD34 and actin. Thoracoscopic lung biopsy also revealed the same histology and these findings were consistent with prostatic STUMP spreading to the lungs (Figure 3B, C). Radical prostatectomy was recommended but the patient refused. For one year of follow up, the patient had no evidence of disease progression.
Prostatic STUMP usually presents in the sixth and seventh decade of life1. The common clinical manifestations are lower urinary tract symptoms, abnormal digital rectal exam, hematuria, and rectal dysfunction. Diagnosis is mainly confirmed by pathologic examination. Based on the degree of stromal cytologic atypia and the presence/appearance of an associated non-neoplastic glandular component, Gaudin et al.1 classified prostatic STUMP into four histological patterns: 1) hypercellular stroma with scattered cytologically atypical cells associated with benign glands, 2) hypercellular stroma with minimal cytological atypia associated with benign glands, 3) hypercellular stroma with or without cytologically atypical cells, associated with benign glands in a leaf like growth pattern that resembles phyllodes tumors of the mammary gland, and 4) hypercelluar stroma without cytologically atypical stromal cells and without glands. By the above classification, the current case belongs to pattern two, because of minimal atypical cells with the presence of benign glands. Previous studies1,4 showed that prostatic STUMP typically express progesterone receptors/CD34, and focally express or do not express desmin. Although CD34 was considered as a useful marker of prostatic STUMP and PSS, there have been some reports7,9 which demonstrated prostatic STUMP with negative immunohistochemical staining for CD34, similar with the findings in our patient. It should be noted that the results of immunohistochemical staining in stromal tumor of the prostate are ancillary and the most important criteria for diagnosis is the morphology by routine Hematoxylin and Eosin stain, as Hansel et al.10 emphasized. Major differential diagnosis includes leiomyosarcoma and rhabdomyosarcoma. Leiomyosarcoma is rare and found in an age distribution similar to prostatic STUMP. The vast majority of leiomyosarcomas exhibit cytologic atypia, high mitotic activity, and occasional foci of coagulative necrosis, whereas there is a lack of mitotic figures and necrosis in prostatic STUMP11. Cheville et al.12 also quotes that leiomyosarcomas display intense cytoplasmic immunoreactivity for actin and vimentin, whereas it reacts weakly with antibodies to desmin. In the current case, the prostatic lesion reveals rare mitotic activity without tumor necrosis and negative immunohistochemical staining for actin. Regarding rhabdomyosarcoma, it is usually found in the first decade of life. The disease can be identified by rhabdomyoblasts in the differentiated tumor and by immunohistochemistry for skeletal muscle markers, such as myogenin or myo-D1 in more primitive tumors10.
Most cases of prostatic STUMP may behave in an indolent manner. Hossain et al.11 insisted prostatic STUMP is a benign, degenerative, and reactive entity, not a neoplastic one. However, local recurrences may occur frequently or rapidly after resection1-4,8. Some cases showed invasion to contiguous organs. In a minority of the cases, prostatic STUMP metastasizes to the lung and bone, and transforms to malignant PSS1,3-7. Intriguingly, by Gaudin's classification, whereas the previous cases are mostly phyllodes tumor in type, the current case is pattern two of prostatic STUMP which spreads to right seminal vesicle and both lungs. In addition, this patient has simultaneously metastatic lung nodules from prostatic STUMP and tuberculous abscess. To our best of knowledge, there have been no case reports about the coexistence of the two diseases. It is thought that the lung lesions might be not associated with tuberculous abscess in the anterior chest. There is no definite treatment guideline in prostatic STUMP, especially with lung metastasis. Considering the neoplastic nature, complete resection of the prostate generally seems to be recommended2. For lung metastasis, Lam and Yeo6 reported the efficacy of chemotherapy with ifosfamide and doxorubicin after radical prostatectomy, in addition to adjuvant irradiation.
In conclusion, the current case shows that prostatic STUMP in pattern 2 invades to the seminal vesicle and spreads to the lungs, supporting the evidence that it is a neoplasm, not an atypical hyperplasia. Considering the risk of recurrence and possibility of progression to PSS, long term follow-up and individualized therapy, which depends on the patient's age, treatment preference, and characteristics of the lesion, are required.
Figures and Tables
Figure 1
Chest computed tomography reveals multiple round nodules scattered in the both lung fields.
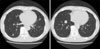
Figure 2
Abdominopelvic computed tomography demonstrates an enlarged prostate with irregular cystic lesions (arrow) and a 2×1 cm sized nodular enhancing mass (arrowhead) in the right seminal vesicle.
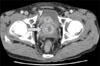
Figure 3
Prostate biopsy shows moderately hypercellular stroma with no cytologic atypia, mitosis or necrosis (A, H&E stain, ×100). The tissues of lung lesion show moderately hypercellular stroma with no cytologic atypia, mitosis or necrosis, which findings are similar with those in prostate (B, H&E stain, ×40, C, H&E stain, ×100).

References
1. Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998. 22:148–162.
2. Wee HM, Ho SH, Tan PH. Recurrent prostatic stromal tumour of uncertain malignant potential (STUMP) presenting with urinary retention 6 years after transurethral resection of prostate (TURP). Ann Acad Med Singapore. 2005. 34:441–442.
3. Bostwick DG, Hossain D, Qian J, Neumann RM, Yang P, Young RH, et al. Phyllodes tumor of the prostate: long-term followup study of 23 cases. J Urol. 2004. 172:894–899.
4. Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006. 30:694–704.
5. Parada D, Ugas G, Peña K, Caricote L, Mujica N. Lung metastases of low grade phyllodes tumor of the prostate: histopathologic confirmation. Arch Esp Urol. 2008. 61:658–662.
6. Lam KC, Yeo W. Chemotherapy induced complete remission in malignant phyllodes tumor of the prostate metastasizing to the lung. J Urol. 2002. 168:1104–1105.
7. Watanabe M, Yamada Y, Kato H, Imai H, Nakano H, Araki T, et al. Malignant phyllodes tumor of the prostate: retrospective review of specimens obtained by sequential transurethral resection. Pathol Int. 2002. 52:777–783.
8. Klausner AP, Unger P, Fine EM. Recurrent prostatic stromal proliferation of uncertain malignant potential: a therapeutic challenge. J Urol. 2002. 168:1493–1494.
9. Roos FC, Sommer S, Hampel C, Melchior SW, Thüroff JW. Extraprostatic spindle cell stromal tumor of the prostate: case report. Urology. 2008. 71:1226.e13-5.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007. 20:148–158.
11. Hossain D, Meiers I, Qian J, MacLennan GT, Bostwick DG. Prostatic stromal hyperplasia with atypia: follow-up study of 18 cases. Arch Pathol Lab Med. 2008. 132:1729–1733.
12. Cheville JC, Dundore PA, Nascimento AG, Meneses M, Kleer E, Farrow GM, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995. 76:1422–1427.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download