Abstract
Background
Most lung cancer patients receive systemic chemotherapy at an advanced stage disease. Cisplatin-based chemotherapy is the main regimen for treating advanced lung cancer. Recently, autophagy has become an important mechanism of cellular adaptation under starvation or cell oxidative stress. The purpose of this study was to determine whether or not autophagy can occurred in cisplatin-treated lung cancer cells.
Methods
H460 cells were incubated with RPMI 1640 and treated in 5 µM or 20 µM cisplatin concentrations at specific time intervals. Cells surviving cisplatin treatment were measured and compared using an MTT cell viability assay to cells that underwent apoptosis with autophagy by nuclear staining, apoptotic or autophagic related proteins, and autophagic vacuoles. The development of acidic vascular organelles was using acridine orange staining and fluorescent expression of GFP-LC3 protein in its transfected cells was observed to evaluate autophagy.
Results
Lung cancer cells treated with 5 µM cisplatin-treated were less sensitive to cell death than 20 µM cisplatin-treated cells in a time-dependent manner. Nuclear fragmentation at 5 µM was not detected, even though it was discovered at 20 µM. Poly (ADP-ribose) polymerase cleavages were not detected in 5 µM within 24 hours. Massive vacuolization in the cytoplasm of 5 µM treated cells were observed. Acridine orange stain-positive cells was increased according in time-dependence manner. The autophagosome-incorporated LC3 II protein expression was increased in 5 µM treated cells, but was not detected in 20 µM treated cells. The expression of GFP-LC3 were increased in 5 µM treated cells in a time-dependent manner.
Figures and Tables
Figure 1
Effect of cisplatin-induced cell death on H460 cells. Tumor cells were seeded at 5×104 cells per well in 48-well flat-bottomed plates and incubated overnight at 37℃. After treated with cisplatin (5 µM or 20 µM) for 3 hr, 6 hr, 12 hr, 24 hr, 48 hr, and 72 hr, the viability of the untreated cells was regarded as 100%. The viability of cell was measured by MTT assay. Control: no cisplatin-treated cells. Means±SD (n=3). *p≤0.05, †p≤0.01.

Figure 2
Cell death induced by cisplatin in NCI-H460 cells did not involve apoptosis. Cells were treated with 5 µM, 20 µM or 5 µM cisplatin with 10 mM 3-MA for 24 hr and assayed for apoptosis by FACS following annexin V-FITC staining. Control cells were treated with RPMI 1640 medium. The data shown are representative of three independent experiments.

Figure 3
Lung cancer cells were treated for several times with 5 µM and 20 µM cisplatin. Western blot analysis showing effects of cisplatin. Western immunoblots probed with antibodies against poly (ADP-ribose) polymerase (PARP) & cleaved PARP and Actin.

Figure 4
Cisplatin induced morphologic changes of H460 cells. Direct observation of cells treated with 5 µM and 20 µM cisplatin for 2 hr, 6 hr, 12 hr, 24 hr, and 48 hr. Scale bar: ×200. Sample were examined under a light microscope.
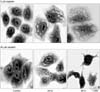
Figure 5
AVOs development in cisplatin-treated H460 cells and its quantificanion using FACS analysis. H460 cells contained RPMI 1640 (control), 5 µM, 20 µM cisplatin or 3-MA with 5 µM cisplatin for 24 hr were stained with acridine orange and analyzed by fluorescent microscopy. Representative images in each group were shown. Note the formation of acridine orange-accumulating AVOs (bright red fluorescence) in cisplatin-treated H460 cells. Similar results were obtained in three independent experiments. *p≤0.01.
References
1. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004. 6:463–477.
2. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004. 23:2891–2906.
3. Schwarze PE, Seglen PO. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen-treated rats. Exp Cell Res. 1985. 157:15–28.
4. Canuto RA, Tessitore L, Muzio G, Autelli R, Baccino FM. Tissue protein turnover during liver carcinogenesis. Carcinogenesis. 1993. 14:2581–2587.
5. Kisen GO, Tessitore L, Costelli P, Gordon PB, Schwarze PE, Baccino FM, et al. Reduced autophagic activity in primary rat hepatocellular carcinoma and ascites hepatoma cells. Carcinogenesis. 1993. 14:2501–2505.
6. Ahlberg J, Yucel T, Eriksson L, Glaumann H. Characterization of the proteolytic compartment in rat hepatocyte nodules. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987. 53:79–88.
7. Yucel T, Ahlberg J, Glaumann H. Overall proteolysis in perfused and subfractionated chemically induced malignant hepatoma of rat: effects of amino acids. Exp Mol Pathol. 1989. 50:38–49.
8. Levine B. Cell biology: autophagy and cancer. Nature. 2007. 446:745–747.
9. Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996. 17:1595–1607.
10. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003. 63:2103–2108.
11. Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001. 61:439–444.
12. Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003. 98:378–384.
13. Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000. 11:49–61.
14. Fram RJ. Cisplatin and platinum analogues: recent advances. Curr Opin Oncol. 1992. 4:1073–1079.
15. Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Autophagy. 2007. 3:610–613.
16. Levine B. Unraveling the role of autophagy in cancer. Autophagy. 2006. 2:65–66.
17. Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009. 1793:1524–1532.
18. Levine B, Abrams J. p53: the Janus of autophagy? Nat Cell Biol. 2008. 10:637–639.
19. Høyer-Hansen M, Jäättelä M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008. 4:574–580.
20. Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007. 3:28–31.
21. Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, et al. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009. 16:87–93.
22. Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007. 6:304–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download