Abstract
Lung cancer remains the most common cause of cancer death in the United States and worldwide. About 80∼90% of cases are smoking-related and smoking cessation programs are of great importance in reducing lung cancer risk. However, the lifetime risk for lung cancer remains elevated even in ex-smokers. Chemoprevention holds the promise to further reduce this risk and thus to decrease lung cancer incidence and mortality. Over the last decades, most chemoprevention trials for lung cancer have yielded negative outcomes. Population-based studies suggest that high intake of certain foods such as soy, red wine or green vegetables may be associated with decreased cancer risk. Because of these observations and their general safety, a plethora of natural compounds is currently being studied for the chemoprevention of cancer. In this review we discuss promising in vitro and in vivo data of novel natural compounds, their interference with molecular mechanisms responsible for lung cancer development and potential implications for their further preclinical and clinical investigation.
Go to : 
REFERENCES
1.American Cancer Society. Global facts and figures 2007-2008. Atlanta: American Cancer Society;2008.
2.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.

3.Doll R., Peto R., Boreham J., Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004. 328:1519.

4.Sporn MB., Dunlop NM., Newton DL., Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. 1976. 35:1332–8.
5.Fisher B., Costantino JP., Wickerham DL., Cecchini RS., Cronin WM., Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005. 97:1652–62.

6.Steinbach G., Lynch PM., Phillips RK., Wallace MH., Hawk E., Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000. 342:1946–52.

7.Thompson IM., Goodman PJ., Tangen CM., Lucia MS., Miller GJ., Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003. 349:215–24.

8.Omenn GS., Goodman GE., Thornquist MD., Balmes J., Cullen MR., Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996. 334:1150–5.

9.Hennekens CH., Buring JE., Manson JE., Stampfer M., Rosner B., Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996. 334:1145–9.

10.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994. 330:1029–35.
11.Tanvetyanon T., Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008. 113:150–7.
12.Hong WK., Lippman SM., Itri LM., Karp DD., Lee JS., Byers RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990. 323:795–801.

13.Lippman SM., Lee JJ., Karp DD., Vokes EE., Benner SE., Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001. 93:605–18.

14.Clark LC., Combs GF Jr., Turnbull BW., Slate EH., Chalker DK., Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial: Nutritional Prevention of Cancer Study Group. JAMA. 1996. 276:1957–63.

15.Reid ME., Duffield-Lillico AJ., Garland L., Turnbull BW., Clark LC., Marshall JR. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002. 11:1285–91.
16.Lippman SM., Klein EA., Goodman PJ., Lucia MS., Thompson IM., Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009. 301:39–51.
18.Häussinger K., Becker H., Stanzel F., Kreuzer A., Schmidt B., Strausz J, et al. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax. 2005. 60:496–503.

19.Khuri FR., Lee JS., Lippman SM., Lee JJ., Kalapurakal S., Yu R, et al. Modulation of proliferating cell nuclear antigen in the bronchial epithelium of smokers. Cancer Epidemiol Biomarkers Prev. 2001. 10:311–8.
20.Kurie JM., Lee JS., Khuri FR., Mao L., Morice RC., Lee JJ, et al. N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res. 2000. 6:2973–9.
21.Lam S., leRiche JC., McWilliams A., Macaulay C., Dyachkova Y., Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004. 10:6502–11.

22.Lam S., MacAulay C., Le Riche JC., Dyachkova Y., Cold-man A., Guillaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002. 94:1001–9.

23.Sato M., Sakurada A., Sagawa M., Minowa M., Takahashi H., Oyaizu T, et al. Diagnostic results before and after introduction of autofluorescence bronchoscopy in patients suspected of having lung cancer detected by sputum cytology in lung cancer mass screening. Lung Cancer. 2001. 32:247–53.

24.Fang MZ., Wang Y., Ai N., Hou Z., Sun Y., Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003. 63:7563–70.
25.Shim JH., Su ZY., Chae JI., Kim DJ., Zhu F., Ma WY, et al. Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev Res (Phila Pa). 2010. 3:670–9.

26.Shanafelt TD., Call TG., Zent CS., LaPlant B., Bowen DA., Roos M, et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009. 27:3808–14.

27.McLarty J., Bigelow RL., Smith M., Elmajian D., Ankem M., Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa). 2009. 2:673–82.

28.Chow HH., Cai Y., Alberts DS., Hakim I., Dorr R., Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001. 10:53–8.
29.Sadava D., Whitlock E., Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007. 360:233–7.

30.Suganuma M., Kurusu M., Suzuki K., Tasaki E., Fujiki H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by up-regulation of GADD153 gene. Int J Cancer. 2006. 119:33–40.
31.Milligan SA., Burke P., Coleman DT., Bigelow RL., Steffan JJ., Carroll JL, et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res. 2009. 15:4885–94.

32.Spitz MR., Duphorne CM., Detry MA., Pillow PC., Amos CI., Lei L, et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000. 9:1017–20.
33.Shapiro TA., Fahey JW., Dinkova-Kostova AT., Holtz-claw WD., Stephenson KK., Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006. 55:53–62.

34.Chiu FL., Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008. 68:61–71.

35.Wu B., Zhang Q., Shen W., Zhu J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem. 2008. 313:125–32.

36.Manju V., Nalini N. Protective role of luteolin in 1,2-di-methylhydrazine induced experimental colon carcinogenesis. Cell Biochem Funct. 2007. 25:189–94.

37.Samy RP., Gopalakrishnakone P., Ignacimuthu S. Antitumor promoting potential of luteolin against 7,12-di-methylbenz(a)anthracene-induced mammary tumors in rats. Chem Biol Interact. 2006. 164:1–14.

38.Thomas MK., Lloyd-Jones DM., Thadhani RI., Shaw AC., Deraska DJ., Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998. 338:777–83.

39.Ingraham BA., Bragdon B., Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008. 24:139–49.

40.Lin J., Manson JE., Lee IM., Cook NR., Buring JE., Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007. 167:1050–9.

41.Menezes RJ., Cheney RT., Husain A., Tretiakova M., Loewen G., Johnson CS, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008. 17:1104–10.

42.Lappe JM., Travers-Gustafson D., Davies KM., Recker RR., Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007. 85:1586–91.

43.Black PN., Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005. 128:3792–8.

44.Horne SL., Cockcroft DW., Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990. 40:173–6.
45.Jang M., Cai L., Udeani GO., Slowing KV., Thomas CF., Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997. 275:218–20.

46.Athar M., Back JH., Tang X., Kim KH., Kopelovich L., Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007. 224:274–83.

47.Banerjee S., Bueso-Ramos C., Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor- kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002. 62:4945–54.
48.Li ZG., Hong T., Shimada Y., Komoto I., Kawabe A., Ding Y, et al. Suppression of N-nitrosomethylbenzyl-amine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002. 23:1531–6.

49.Revel A., Raanani H., Younglai E., Xu J., Rogers I., Han R, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003. 23:255–61.

50.Hecht SS., Kenney PM., Wang M., Trushin N., Agarwal S., Rao AV, et al. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999. 137:123–30.

51.Berge G., Ovrebo S., Eilertsen E., Haugen A., Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br J Cancer. 2004. 91:1380–3.

52.Walle T., Hsieh F., DeLegge MH., Oatis JE Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004. 32:1377–82.

53.Boocock DJ., Faust GE., Patel KR., Schinas AM., Brown VA., Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007. 16:1246–52.

54.Pongrakhananon V., Nimmannit U., Luanpitpong S., Ro-janasakul Y., Chanvorachote P. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis. 2010. 15:574–85.

55.Su CC., Yang JS., Lu CC., Chiang JH., Wu CL., Lin JJ, et al. Curcumin inhibits human lung large cell carcinoma cancer tumour growth in a murine xenograft model. Phytother Res. 2010. 24:189–92.

56.Chen Q., Wang Y., Xu K., Lu G., Ying Z., Wu L, et al. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol Rep. 2010. 23:397–403.

57.Cheng AL., Hsu CH., Lin JK., Hsu MM., Ho YF., Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or premalignant lesions. Anticancer Res. 2001. 21:2895–900.
58.Cruz-Correa M., Shoskes DA., Sanchez P., Zhao R., Hylind LM., Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006. 4:1035–8.
59.Somers-Edgar TJ., Scandlyn MJ., Stuart EC., Le Nedelec MJ., Valentine SP., Rosengren RJ. The combination of epigallocatechin gallate and curcumin suppresses ER alpha-breast cancer cell growth in vitro and in vivo. Int J Cancer. 2008. 122:1966–71.
60.Khafif A., Schantz SP., Chou TC., Edelstein D., Sacks PG. Quantitation of chemopreventive synergism between (-)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis. 1998. 19:419–24.
61.Li Y., Kucuk O., Hussain M., Abrams J., Cher ML., Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of os-teoprotegerin/receptor activator of nuclear factor-ka-ppaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006. 66:4816–25.
62.Hillman GG., Wang Y., Kucuk O., Che M., Doerge DR., Yudelev M, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004. 3:1271–9.
63.Hussain M., Banerjee M., Sarkar FH., Djuric Z., Pollak MN., Doerge D, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003. 47:111–7.

64.Zou H., Zhan S., Cao K. Apoptotic activity of genistein on human lung adenocarcinoma SPC-A-1 cells and preliminary exploration of its mechanisms using microarray. Biomed Pharmacother. 2008. 62:583–9.

65.Gadgeel SM., Ali S., Philip PA., Wozniak A., Sarkar FH. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009. 115:2165–76.
66.Shimazu T., Inoue M., Sasazuki S., Iwasaki M., Sawada N., Yamaji T, et al. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. Am J Clin Nutr. 2010. 91:722–8.

67.Lynch TJ., Bell DW., Sordella R., Gurubhagavatula S., Okimoto RA., Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–39.

68.Paez JG., Jänne PA., Lee JC., Tracy S., Greulich H., Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–500.
69.Soda M., Choi YL., Enomoto M., Takada S., Yamashita Y., Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007. 448:561–6.

70.Herman JG., Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003. 349:2042–54.

71.Belinsky SA., Palmisano WA., Gilliland FD., Crooks LA., Divine KK., Winters SA, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002. 62:2370–7.
72.McCabe MT., Brandes JC., Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009. 15:3927–37.

73.Damiani LA., Yingling CM., Leng S., Romo PE., Nakamura J., Belinsky SA. Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bronchial epithelial cells. Cancer Res. 2008. 68:9005–14.

74.Belinsky SA., Klinge DM., Stidley CA., Issa JP., Herman JG., March TH, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003. 63:7089–93.
75.Majid S., Dar AA., Shahryari V., Hirata H., Ahmad A., Saini S, et al. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer. 2010. 116:66–76.

76.Fang MZ., Chen D., Sun Y., Jin Z., Christman JK., Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005. 11:7033–41.
77.Majid S., Kikuno N., Nelles J., Noonan E., Tanaka Y., Kawamoto K, et al. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Res. 2008. 68:2736–44.

78.Schmid K., Oehl N., Wrba F., Pirker R., Pirker C., Filipits M. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res. 2009. 15:4554–60.

79.Mok TS., Wu YL., Thongprasert S., Yang CH., Chu DT., Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–57.

80.Pao W., Wang TY., Riely GJ., Miller VA., Pan Q., Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005. 2:e17.

81.Rusch V., Klimstra D., Linkov I., Dmitrovsky E. Aberrant expression of p53 or the epidermal growth factor receptor is frequent in early bronchial neoplasia and co-expression precedes squamous cell carcinoma development. Cancer Res. 1995. 55:1365–72.
82.Lev-Ari S., Starr A., Vexler A., Karaush V., Loew V., Greif J, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, downregulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006. 26:4423–30.
83.Lee LT., Huang YT., Hwang JJ., Lee AY., Ke FC., Huang CJ, et al. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004. 67:2103–14.

84.Lee LT., Huang YT., Hwang JJ., Lee PP., Ke FC., Nair MP, et al. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002. 22:1615–27.
85.Huang YT., Hwang JJ., Lee PP., Ke FC., Huang JH., Huang CJ, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpres-sing epidermal growth factor receptor. Br J Pharmacol. 1999. 128:999–1010.

86.Syed DN., Afaq F., Kweon MH., Hadi N., Bhatia N., Spiegelman VS, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007. 26:673–82.
87.Zhang Q., Kelly AP., Wang L., French SW., Tang X., Duong HS, et al. Green tea extract and (-)-epigal-locatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol. 2006. 126:2607–13.

88.Beevers CS., Chen L., Liu L., Luo Y., Webster NJ., Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009. 69:1000–8.

89.Yu S., Shen G., Khor TO., Kim JH., Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008. 7:2609–20.

90.Beevers CS., Li F., Liu L., Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer. 2006. 119:757–64.

91.Jiang H., Shang X., Wu H., Gautam SC., Al-Holou S., Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009. 8:25–33.
92.Brito PM., Devillard R., Nègre-Salvayre A., Almeida LM., Dinis TC., Salvayre R, et al. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009. 205:126–34.

93.Nakamura Y., Yogosawa S., Izutani Y., Watanabe H., Otsuji E., Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer. 2009. 8:100.

94.Anastasius N., Boston S., Lacey M., Storing N., Whitehead SA. Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol. 2009. 116:50–5.

95.Khan N., Afaq F., Kweon MH., Kim K., Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007. 67:3475–82.

96.Tang FY., Cho HJ., Pai MH., Chen YH. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J Nutr Biochem. 2009. 20:426–34.

97.Licchesi JD., Westra WH., Hooker CM., Machida EO., Baylin SB., Herman JG. Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis. 2008. 29:895–904.

98.Nguyen DX., Chiang AC., Zhang XH., Kim JY., Kris MG., Ladanyi M, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009. 138:51–62.

99.Gao Z., Xu Z., Hung MS., Lin YC., Wang T., Gong M, et al. Promoter demethylation of WIF-1 by epigallocat-echin-3-gallate in lung cancer cells. Anticancer Res. 2009. 29:2025–30.
100.Kim J., Zhang X., Rieger-Christ KM., Summerhayes IC., Wazer DE., Paulson KE, et al. Suppression of Wnt signaling by the green tea compound (-)-epigallocat-echin 3-gallate (EGCG) in invasive breast cancer cells: requirement of the transcriptional repressor HBP1. J Biol Chem. 2006. 281:10865–75.
101.Pahlke G., Ngiewih Y., Kern M., Jakobs S., Marko D., Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006. 54:7075–82.

102.Kakarala M., Brenner DE., Korkaya H., Cheng C., Tazi K., Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010. 122:777–85.

103.Jaiswal AS., Marlow BP., Gupta N., Narayan S. Beta-cat-enin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmeth-ane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002. 21:8414–27.
104.Ryu MJ., Cho M., Song JY., Yun YS., Choi IW., Kim DE, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun. 2008. 377:1304–8.
105.Choi H., Gwak J., Cho M., Ryu MJ., Lee JH., Kim SK, et al. Murrayafoline A attenuates the Wnt/beta-catenin pathway by promoting the degradation of intracellular beta-catenin proteins. Biochem Biophys Res Commun. 2010. 391:915–20.
106.Li Y., Zhang T., Korkaya H., Liu S., Lee HF., Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010. 16:2580–90.

107.Ramos-Gomez M., Kwak MK., Dolan PM., Itoh K., Yamamoto M., Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001. 98:3410–5.

108.Fahey JW., Haristoy X., Dolan PM., Kensler TW., Scholtus I., Stephenson KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a] pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002. 99:7610–5.
109.Pereira FM., Rosa E., Fahey JW., Stephenson KK., Carvalho R., Aires A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea Var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agric Food Chem. 2002. 50:6239–44.

110.Thimmulappa RK., Rangasamy T., Alam J., Biswal S. Dibenzoylmethane activates Nrf2-dependent detoxification pathway and inhibits benzo(a)pyrene induced DNA adducts in lungs. Med Chem. 2008. 4:473–81.

111.Iida K., Itoh K., Kumagai Y., Oyasu R., Hattori K., Kawai K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004. 64:6424–31.

112.Kelley MJ., Glaser EM., Herndon JE 2nd., Becker F., Bhagat R., Zhang YJ, et al. Safety and efficacy of weekly oral oltipraz in chronic smokers. Cancer Epidemiol Biomarkers Prev. 2005. 14:892–9.

113.Dilman VM., Berstein LM., Zabezhinski MA., Alexandrov VA., Bobrov JF., Pliss GB. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Arch Geschwulstforsch. 1978. 48:1–8.
114.Evans JM., Donnelly LA., Emslie-Smith AM., Alessi DR., Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005. 330:1304–5.

115.Zakikhani M., Dowling R., Fantus IG., Sonenberg N., Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006. 66:10269–73.

116.Rattan R., Giri S., Singh AK., Singh I. 5-Aminoimida-zole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005. 280:39582–93.
117.Hwang JT., Ha J., Park IJ., Lee SK., Baik HW., Kim YM, et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007. 247:115–21.

118.Collins QF., Liu HY., Pi J., Liu Z., Quon MJ., Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J Biol Chem. 2007. 282:30143–9.

119.Hwang JT., Park IJ., Shin JI., Lee YK., Lee SK., Baik HW, et al. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2005. 338:694–9.

120.Fan E., Jiang S., Zhang L., Bai Y. Molecular mechanism of apoptosis induction by resveratrol, a natural cancer chemopreventive agent. Int J Vitam Nutr Res. 2008. 78:3–8.

121.Breen DM., Sanli T., Giacca A., Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sir-tuins and AMPK. Biochem Biophys Res Commun. 2008. 374:117–22.

122.Park CE., Kim MJ., Lee JH., Min BI., Bae H., Choe W, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007. 39:222–9.

123.Pan W., Yang H., Cao C., Song X., Wallin B., Kivlin R, et al. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep. 2008. 20:1553–9.

124.Ahn J., Lee H., Kim S., Park J., Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008. 373:545–9.

125.Hwang JT., Kim SH., Lee MS., Yang HJ., Kim MJ., Kim HS, et al. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2007. 364:1002–8.

Go to : 
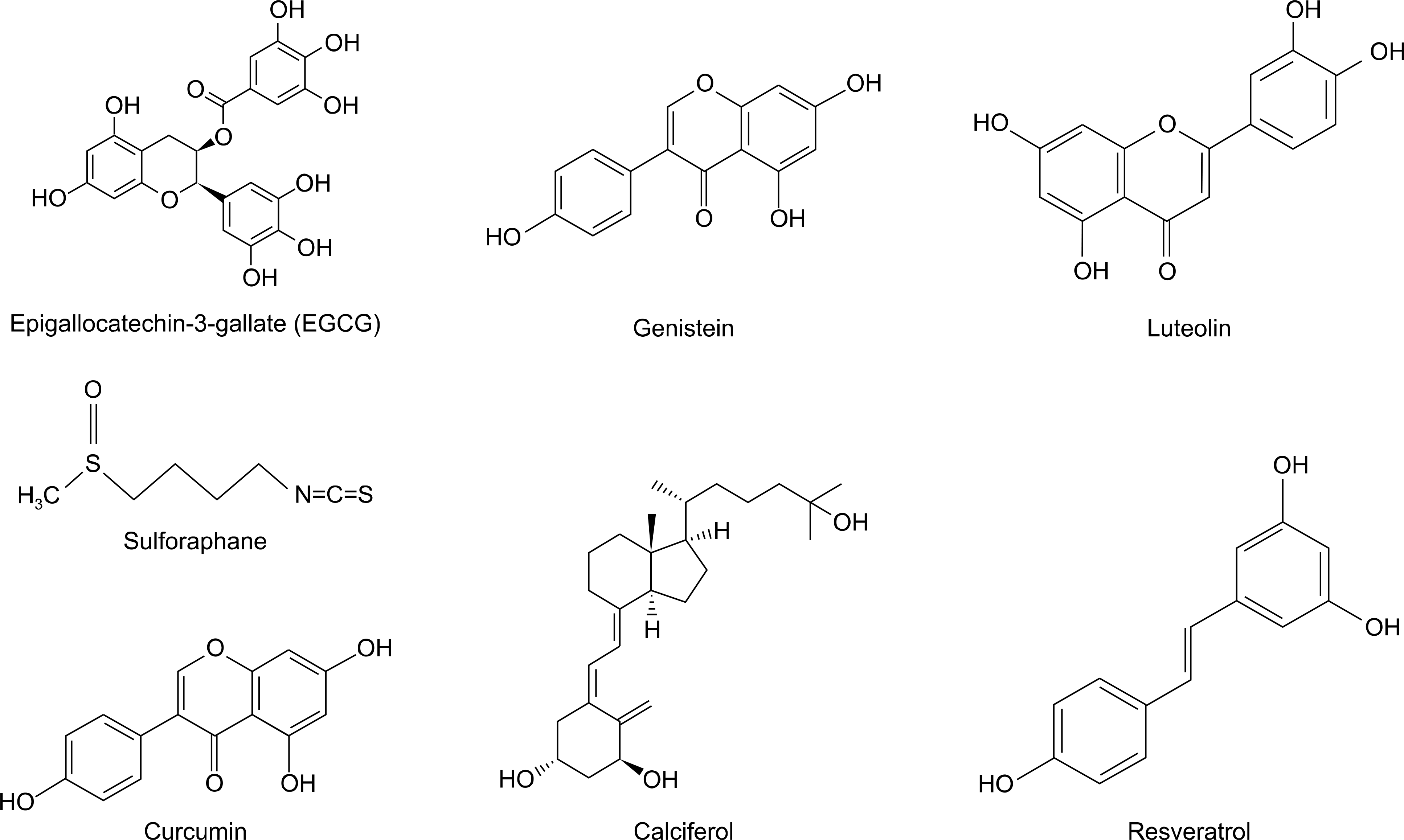 | Figure 1.Structures of phytochemical compounds with potential for lung cancer chemoprevention. |
Table 1.
Phytochemicals with potential lung cancer chemopreventive effects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download