Abstract
Here we report the first fatality caused by H1N1 influenza virus infection with acute respiratory distress syndrome in Korea. A 55-year-old man presented at our emergency department with dyspnea, fever, diffuse myalgia and malaise. Bilateral lung air-space consolidation was detected on his initial chest radiograph combined with severe hypoxemia. He was supported by mechanical ventilation and treated with antibiotics. A nasopharyngeal aspirate was positive for influenza A rapid antigen and oseltamivir was started on day 3 of admission. The nasal swab sample was positive for influenza H1N1 virus by real-time reverse-transcriptase polymerase chain reaction. Despite aggressive treatment, he had refractory hypoxemia and uncontrolled septic shock. On day 5 of admission he went into cardiac arrest and expired.
A novel influenza H1N1 strain emerged in Mexico in late March 2009. As of 6 December 2009, worldwide more than 208 countries and overseas territories or communities have reported laboratory confirmed cases of pandemic influenza H1N1 2009, including almost 10,000 deaths1. As of 16 December 2009, South Korea has reported over 130 fatal cases since a 51-year-old woman was first confirmed to have H1N1 infection in May 20092. This report describes the first case of novel influenza A (H1N1) fatality in South Korea.
A 55-year-old previously healthy man was admitted to our emergency department (ED), in August 2009 with dyspnea, fever, diffuse myalgia and malaise. However, he didn't have any respiratory symptoms such as cough, sputum, sore throat and rhinorrhea except dyspnea. Five days earlier, he had returned from a vacation in Thailand. Febrile sensation had started two days earlier and diffuse myalgia one day earlier. When he visited a local hospital one day earlier, his chest radiograph showed airspace opacification and he was treated with oxygen and antibiotics but his condition deteriorated rapidly. He required intubation and referred to our ED. He had no significant medical history other than benign prostatic hyperplasia. He had smoked cigarettes, half pack per day, for more than 30 years.
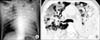
On admission, he had semicomatous mentality and no spontaneous breathing, with a blood pressure of 110/70 mm Hg, heart rate of 126 beats/min, and a temperature of 36.5℃. Crackles were heard bilaterally in his upper lung fields. There were bilateral air-space consolidation on the initial chest radiograph and also bilateral peripheral consolidation and ground-glass opacity with centrilobular nodules on computed tomography scans (Figure 1). Arterial blood gas analysis gave pH 7.349, PaO2 32.7 mm Hg, PaCO2 40.1 mm Hg, SaO2 57.8% (10 L/min of oxygen with ambu mask). He was supported by mechanical ventilation and admitted directly to the intensive care unit (ICU).
Laboratory data on admission were as follows: white blood cell count 6,560/µL with 95.7% segmental neutrophil and 2.9% lymphocyte, hemoglobin 11.9 g/dL, hematocrit 34.5%, platelet 134,000/µL, and C-reactive protein (CRP) 38.36 mg/dL. Prothrombin and partial thromboplastin times were normal. Liver function test results were: total protein 6.2 g/dL, albumin 3.4 g/dL, total bilirubin 1.07 mg/dL, direct bilirubin 0.35 mg/dL, aspartate aminotransferase (AST) 93 IU/L, alanine aminotransferase (ALT) 50 IU/L, alkaline phosphatase 179 IU/L, and lactate dehydrogenase (LDH) 799 IU/L. Furthermore, blood urea nitrogen (BUN) was 18.5 mg/dL, creatinine 1.2 mg/dL, creatine kinase (CK) 844 U/L, serum sodium 132.5 mEq/L, and potassium 3.94 mEq/L. Urine streptococcal and legionella antigens were negative. Gram-stained smears and cultures of sputum and blood cultures had negative results.
Under a presumptive diagnosis of bacterial pneumonia with acute respiratory distress syndrome (ARDS), the patient received intravenous meropenem and levofloxacin. His blood pressure dropped to 80/40 mm Hg and urine output was decreased on the next day. A nasopharyngeal aspirate (NPA) was positive for influenza A rapid antigen and oseltamivir (75 mg twice daily) was started on day 3 of admission. The nasal swab sample was positive for influenza H1N1 virus by the real-time reverse-transcriptase polymerase chain reaction (RT-PCR). He had refractory hypoxemia with a PaO2 down to 60 mm Hg while receiving ventilator support of fraction of inspired oxygen (FiO2) 1.0 at positive end-expiratory pressure of 20 cm H2O. Despite vigorous hydration and inotropics, the septic shock was out of control. On day 4 of admission he required continuous renal replacement therapy for acute renal failure. On day 5 of admission he went into cardiac arrest and expired. This is the first novel influenza A (H1N1) fatality case, confirmed by The Korea Centers for Disease Control and Prevention. None of his family members and coworkers was confirmed to have influenza H1N1 infection.
A swine-origin influenza A (H1N1) virus (S-OIV) is characterized by a unique combination of gene segments that has not been previously identified among human or swine influenza A viruses. In fact, the new H1N1 virus appears to be a mixture of avian, porcine, and human influenza RNA3,4. This provokes an almost universal vulnerability to infection in nearly all people. Particularly the younger age-groups are much more susceptible than the elderly to H1N1 infection. This is consistent with the age-stratified sero-epidemiologic data, which suggests that persons above 60 years of age are more likely to have neutralizing antibodies to the virus5.
The H1N1 virus infection usually causes an acute, self-limiting, febrile illness, however, it can also lead to severe outcomes, including respiratory failure and death. In severe cases the most common finding is primary viral pneumonia, which is a frequent cause of death. Secondary bacterial infections have been found in approximately 30% of fatal cases. Respiratory failure and refractory shock have been the most common causes of death6.
The clinical picture in severe cases seems markedly different from the disease pattern seen during epidemics of seasonal influenza. While people with certain underlying medical conditions, including pregnancy, are known to be at increased risk, many severe cases occur in previously healthy young people. In these patients, predisposing factors that increase the risk of severe illness are not presently understood, though research is under way6,7.
In severe cases, patients generally begin to deteriorate about 3 to 5 days after symptom onset. Deterioration is rapid, with many patients progressing to respiratory failure within 24 hours, requiring immediate admission to an ICU, associated with high (17~58%) mortality 7-10.
This patient was suspected to have bacterial pneumonia with ARDS at first because of fever, an elevated level of CRP, and bilateral lung air-space consolidation on his chest radiograph with severe hypoxemia. Urine streptococcal and legionella antigens were negative. While sputum and blood cultures had negative results, NPA was positive for influenza A rapid antigen, as was the RT-PCR for influenza H1N1 and H1N1 virus infection was confirmed.
In the United States from April 2009 to mid-June 2009, Jain et al.11 reported clinical characteristics of patients who were hospitalized with novel influenza A (H1N1). Of the 249 patients, 100 (40%) had findings that were consistent with pneumonia; 162 (60%) had shortness of breath; 66% had an underlying medical condition. A study by Perez-Padilla et al.7 reported that common findings of 18 persons with pneumonia and H1N1 infection were increased serum LDH and CK levels and lymphopenia and all of their radiographs revealed bilateral patchy alveolar opacities. These laboratory and radiologic findings were also found in our case. Therefore it is necessary to suspect H1N1 influenza infection when a previously healthy young person presents signs and symptoms such as fever, myalgia and dyspnea with bilateral patchy alveolar opacities on the chest radiograph and increased serum LDH and CK levels and lymphopenia in laboratory studies.
Figures and Tables
References
1. Pandemic (H1N1) 2009: update 78 [Internet]. World Health Organization. 2009. cited 2009 Dec 18. Geneva: World Health Organization;Available from:
http://www.who.int/csr/don/2009_12_11a/en/index.html.
2. pandemic (H1N1) 2009: ECDC daily update [Internet]. ECDC. 2009. cited 2009 Dec 18. Stockholm: European Centre for Disease Prevention and Control;Available from:
http://ecdc.europa.eu/en/healthtopics/Pages/Influenza_A(H1N1)_Outbreak.aspx.
3. Trifonov V, Khiabanian H, Greenbaum B, Rabadan R. The origin of the recent swine influenza A (H1N1) virus infecting humans. Euro Surveill. 2009. 14:pii:19193.
4. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009. 360:2605–2615.
5. Centers for Disease Control and Prevention (CDC). Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009. 58:521–524.
6. Clinical features of severe cases of pandemic influenza. Pandemic (H1N1) 2009 briefing note 13 [Internet]. World Health Organization. 2009. cited 2009 Dec 21. Geneva: World Health Organization;Available from:
http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html.
7. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009. 361:680–689.
8. Centers for Disease Control and Prevention (CDC). Intensive-care patients with severe novel influenza A (H1N1) virus infection: Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:749–752.
9. Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009. 13.
10. Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009. 302:1872–1879.
11. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009. 361:1935–1944.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download