Abstract
Background
Based on the known immunoregulatory functions of moxifloxacin on phagocytes, the therapeutic effect of moxifloxacin on oleic acid (OA)-induced acute lung injury (ALI) was investigated.
Methods
Moxifloxacin (10 mg/kg) was given to male Sprague-Dawley rats that had been given oleic acid (OA, 30 µL) intravenously. Five hours after OA injection, parameters demonstrating ALI were assessed to measure the effects of moxifloxacin on acute lung injury.
Results
The pathological findings of OA-induced ALI's was diminished by moxifloxacin. Through ultrastructural and CeCl3 EM histochemistry, moxifloxacin was confirmed to be effective in decreasing oxidative stress in the lung as well. Indices of ALI, such as lung weight/body weight ratio, protein content in bronchoalveolar lavage fluid, and lung myeloperoxidase were decreased by moxifloxacin. In diaminobenzidine immunohistochemistry, fluorescent immunohistochemistry, and Western blotting of the lung, moxifloxacin had decreased the enhanced expression of secretory phospholipase A2 (sPLA2) by OA.
Figures and Tables
Figure 1
(A) The effect of oleic acid on the morphological changes in the lung. Intraalveolar phagocytes, especially neutrophils were found abundantly. Hyaline membrane and wide spread hemorrhage were found also (H&E stain, ×40). (B) The effect of moxifloxacin on the histological changes in the lung of rat given oleic acid intravenously. Alveoli are patent and the accumulation and migration of neutrophils into the alveolar lumen were significantly lessened by moxifloxacin. Generalized inflammatory changes were diminished by moxifloxacin (H&E stain, ×100).

Figure 2
The ultrastructural changes of alveolar type II cells of the lung in oleic acid (OA), OA with moxifloxacin treated rats. Well preserved lamellar bodies were found in alveolar type II cells of the control lung (A). In OA treated rat, a vacuolization of lamellar bodies of alveolar type II cells was prominent, a direct evidence of oxidative stress (B). In contrast, OA with moxifloxacin treated rats, lamellar bodies of alveolar type II cells were relatively well preserved (C) (Original magnification ×3,000).
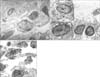
Figure 3
The detection of hydrogen peroxide with CeCl3 cytochemical electro microscopy. In control and oleic acid with moxifloxacin rats, few cerrous perhydroxide granules were found in the vicinity of alveolar type II cells (A, C). In contrast, dense deposits of cerrous perhydroxide granules were found along the membrane of alveolar type II cell (B) (Original magnification ×3,000).
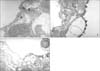
Figure 4
(A) An immunohistochemistry for the detection of secretory phospholipase A2 (sPLA2) in the normal rat's lung. Staining sPLA2 with diaminobenzidine revealed few reactive cells against sPLA2 antibody. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200). (B) A representative of immunohistochemistry for detection of sPLA2 in the lung of oleic acid (OA) given rat. In the alveoli, abundant migrated neutrophils of which cytoplasm and cell membrane were intensely reacted with diaminobenzidine signifying strong activation of sPLA2 in these cells. Hyaline membranes were found also in the alveolar lumen. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200). (C) An immunohistochemical photograph of detecting sPLA2 in the lung of rat given OA and moxifloxacin. Few phagocytes were found in the lung and diaminobenzidine positive cell was not found. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200).

Figure 5
(A) A fluorescent immunohistochemistry for detection of secretory phospholipase A2 (sPLA2) in the normal rat's lung. Counter-staining with propidium iodide of sPLA2 revealed few cells of sPLA2 positive against sPLA2 antibody. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200). (B) A representative of fluorescent immunohistochemistry for detection of sPLA2 in the lung of oleic acid (OA) given rat. Dense, highly fluorescent area were found in the lung depicting the strong expression of PLA2 in the lung. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200). (C) A fluorescent immunohistochemical photograph of detecting sPLA2 in the lung of rat given OA and moxifloxacin. Fluorescent area of lung by OA was strikingly diminished by moxifloxacin signifying the inhibition of PLA2. Primary antibody against sPLA2 was goat anti-human sPLA2 polyclonal antibody (dilutional factor ×50) and secondary antibody was biotinylated anti-goat IgG (dilutional factor ×100) (Original magnification ×200).
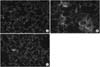
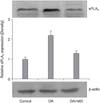
Figure 6
Western blot analysis of secretory phospholipase A2 (sPLA2) in the lung. Oleic acid (OA) treatment increased the expression of sPLA2 but this increased expression was down-regulated by moxifloxacin (MO).
References
1. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.
2. Pruzanski W, Vadas P. Phospholipase A2: a mediator between proximal and distal effectors of inflammation. Immunol Today. 1991. 12:143–146.
3. Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997. 155:1187–1205.
4. Chenevier-Gobeaux C, Simonneau C, Therond P, Bonnefont-Rousselot D, Poiraudeau S, Ekindjian OG, et al. Implication of cytosolic phospholipase A2 (cPLA2) in the regulation of human synoviocyte NADPH oxidase (Nox2) activity. Life Sci. 2007. 81:1050–1058.
5. Dana R, Malech HL, Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994. 297(Pt 1):217–223.
6. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006. 1761:1246–1259.
7. Lee YM, Hybertson BM, Cho HG, Terada LS, Cho O, Repine AJ, et al. Platelet-activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L75–L80.
8. Martensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991. 88:9360–9364.
9. Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, Maekawa N, et al. Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits. Am J Respir Crit Care Med. 1999. 160:1292–1302.
10. Werber S, Shalit I, Fabian I, Steuer G, Weiss T, Blau H. Moxifloxacin inhibits cytokine-induced MAP kinase and NF-kappaB activation as well as nitric oxide synthesis in a human respiratory epithelial cell line. J Antimicrob Chemother. 2005. 55:293–300.
11. Weiss T, Shalit I, Blau H, Werber S, Halperin D, Levitov A, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob Agents Chemother. 2004. 48:1974–1982.
12. Uriarte SM, Molestina RE, Miller RD, Bernabo J, Farinati A, Eiguchi K, et al. Effects of fluoroquinolones on the migration of human phagocytes through Chlamydia pneumoniae-infected and tumor necrosis factor alpha-stimulated endothelial cells. Antimicrob Agents Chemother. 2004. 48:2538–2543.
13. Shalit I, Horev-Azaria L, Fabian I, Blau H, Kariv N, Shechtman I, et al. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob Agents Chemother. 2002. 46:2442–2449.
14. Shiue ST, Thrall RS. Effect of corticosteroid therapy on the acute injury and recovery stage of oleic acid induced lung injury in the rat. Exp Lung Res. 1991. 17:629–638.
15. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989. 180:136–139.
16. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985. 59:1978–1985.
17. Hobson J, Wright J, Churg A. Histochemical evidence for generation of active oxygen species on the apical surface of cigarette-smoke-exposed tracheal explants. Am J Pathol. 1991. 139:573–580.
18. Matalon S, Ji HL. Oleic acid damages ion transport and promotes alveolar edema: the dark side of healthy living. Am J Respir Crit Care Med. 2005. 171:424–425.
19. Lee YM, Kim BY, Park YY. Role of the PLA2-activated neutrophilic oxidative stress in oleic acid-induced acute lung injury. Tuberc Respir Dis. 2010. 68:55–61.
20. Williams AC, Galley HF, Webster NR. The effect of moxifloxacin on release of interleukin-8 from human neutrophils. Br J Anaesth. 2001. 87:671–672.
21. Zimmermann GS, Neurohr C, Villena-Hermoza H, Hatz R, Behr J. Anti-inflammatory effects of antibacterials on human Bronchial epithelial cells. Respir Res. 2009. 10:89.
22. Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007. 76:44–50.
23. Ljungman AG, Tagesson C, Lindahl M. Endotoxin stimulates the expression of group II PLA2 in rat lung in vivo and in isolated perfused lungs. Am J Physiol. 1996. 270.
24. Arbibe L, Vial D, Rosinski-Chupin I, Havet N, Huerre M, Vargaftig BB, et al. Endotoxin induces expression of type II phospholipase A2 in macrophages during acute lung injury in guinea pigs: involvement of TNF-alpha in lipopolysaccharide-induced type II phospholipase A2 synthesis. J Immunol. 1997. 159:391–400.
25. Lindbom J, Ljungman AG, Lindahl M, Tagesson C. Increased gene expression of novel cytosolic and secretory phospholipase A(2) types in human airway epithelial cells induced by tumor necrosis factor-alpha and IFN-gamma. J Interferon Cytokine Res. 2002. 22:947–955.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download