Abstract
Background
Sepsis still has a high mortality rate despite adequate supportive care. Newer therapeutic modalities have been developed but they have generally ended in failure. Recently, insulin was reported to have an anti-inflammatory effect by inhibiting the IκB/NF-κB pathway, and may have therapeutic potential in sepsis. However, the precise mechanism of the anti-inflammatory effect of insulin is unclear. This study examined the role of insulin in activating IκB/NF-κB in macrophage.
Methods
Raw 264.7 cells, a murine macrophage cell line, were used in this experiment. Western blotting using IκB Ab and phosphor-specific IκB Ab was performed to evaluate the degradation and phosphorylation of IκB cells. For the IκB Kinase (IKK) activity, an immune complex kinase assay was performed. The level of interleukin-6 (IL-6) was measured by ELISA to determine the level of proinflammatory cytokine.
Results
IκBα degradation began 30 min after lipopolysaccharide (LPS) treatment. However, an insulin pretreatment suppressed the IκBα degradation caused by the LPS treatment. The phosphorylation of IκBα and IKK activity was also inhibited by the insulin pretreatment. Finally, the insulin pretreatment showed a tendency to suppress the induction of IL-6 by LPS.
Figures and Tables
Figure 1
Insulin suppresses lipopolysaccharide (LPS)-induced IκBα degradation in macrophage cell line. (A) Raw 264.7 cells were pre-treated with 10 µg/mL of insulin for 6 hours and then treated with 1 µg/mL of LPS for 0, 0.5, 2, 4 hours. (B) Raw 264.7 cells were pre-treated with 10 µg/mL of insulin for 0, 2, 4, 24 hours and then treated with 1 µg/mL of LPS for 30 minutes. IκBα and actin expressions were evaluated by Western blot. Results are representative of three distinct experiments.

Figure 2
Insulin suppresses lipopolysaccharide (LPS)-induced IκBα phosphorylation in macrophage cell line. Raw 264.7 cells were pre-treated with 10 µg/mL of insulin for 6 hours and then treated with 1 µg/mL of LPS for 0, 5, 10, 30 minutes. Phosphorylated IκBα and actin expressions were evaluated by Western blot. Results are representative of three distinct experiments.
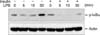
Figure 3
Insulin attenuates lipopolysaccharide (LPS)-induced p65 in macrophage cell line. Raw 264.7 cells were pre-treated with 10 µg/mL of insulin for 6 hours and then treated with 1 µg/mL of LPS for 0, 10, 30 minutes. Phosphorylated p65 and actin expressions were evaluated by Western blot. Results are representative of three distinct experiments.

Figure 4
Insulin inhibits lipopolysaccharide (LPS)-induced IκB kinase (IKK) activation in macrophage cell line. Raw 264.7 cells were pre-treated with 10 µg/mL of insulin for 6 hours and then treated with 1 µg/mL of LPS for 5 minutes. IKK activity was evaluated by in vitro immune complex kinase assay. Results are representative of three distinct experiments.
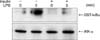
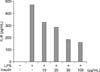
Figure 5
Insulin attenuates lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) secretion dose-dependently in macrophage cell line. Raw 264.7 cells were pre-treated with 0, 10, 20, 30, 100 µg/mL of insulin for 6 hours and then treated with 1 µg/mL of LPS for 24 hours. Levels of secreted IL-6 were evaluated by ELISA. Results are representative of three distinct experiments.
References
1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001. 29:1303–1310.
2. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987. 317:653–658.
3. Ziegler EJ, Fisher CJ Jr, Sprung CL, Straube RC, Sadoff JC, Foulke GE, et al. The HA-1A Sepsis Study Group. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin: a randomized, double-blind, placebo-controlled trial. N Engl J Med. 1991. 324:429–436.
4. Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, et al. The Soluble TNF Receptor Sepsis Study Group. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. N Engl J Med. 1996. 334:1697–1702.
5. Fisher CJ Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994. 22:12–21.
6. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001. 345:1359–1367.
7. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002. 87:978–982.
8. Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003. 31:359–366.
9. Martins JO, Ferracini M, Ravanelli N, Landgraf RG, Jancar S. Insulin suppresses LPS-induced iNOS and COX-2 expression and NF-kappaB activation in alveolar macrophages. Cell Physiol Biochem. 2008. 22:279–286.
10. Martins JO, Ferracini M, Ravanelli N, Landgraf RG, Jancar S. Insulin inhibits LPS-induced signaling pathways in alveolar macrophages. Cell Physiol Biochem. 2008. 21:297–304.
11. Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988. 242:540–546.
12. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996. 87:13–20.
13. Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995. 80:529–532.
14. Aljada A, Ghanim H, Saadeh R, Dandona P. Insulin inhibits NFkappaB and MCP-1 expression in human aortic endothelial cells. J Clin Endocrinol Metab. 2001. 86:450–453.
15. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001. 86:3257–3265.
16. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008. 358:125–139.
17. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006. 354:449–461.
18. Baeuerle PA, Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989. 3:1689–1698.
19. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994. 12:141–179.
20. Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab. 2000. 85:2572–2575.
21. Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007. 148:346–353.
22. Barkhausen T, Probst C, Hildebrand F, Pape HC, Krettek C, van Griensven M. Insulin therapy induces changes in the inflammatory response in a murine 2-hit model. Injury. 2009. 40:806–814.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download