Abstract
Background
To analyze the result of 18F-FDG positron emission tomography (PET) in patients with a concomitant malignancy and tuberculoma in a tuberculosis (TB)-endemic area.
Methods
Twelve patients with a concomitant malignancy and tuberculoma, who underwent whole-body 18F-FDG PET, were evaluated retrospectively. The maximal standardized uptake values (SUVmax) of the malignancy and tuberculoma were compared. In 6 patients, 18F-FDG PET was repeated during the anti-TB treatment and the changes in SUVmax were analyzed.
Results
Of the 12 patients, 10 were male. The mean age was 67.2±7.9 years. Tuberculomas were located in the lung (n=10) and lymph nodes (n=2), and tumors were located in the lung (n=6), colon (n=3), stomach (n=1), ovary (n=1) and liver (n=1). Although the mean SUVmax of malignant lesions was higher than that of tuberculomas (5.2±3.2 vs 3.5±2.0), the difference was not significant. In 4 patients, the SUVmax was higher in the tuberculoma than the tumor. After anti-TB treatment in 6 patients, the mean SUVmax of the tuberculomas decreased significantly, from 3.5±2.0 to 1.6±0.9 (p=0.028).
[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET) is a functional imaging technique that monitors glucose metabolism in tissues. 18F-FDG-PET has been used to differentiate malignant tumors from benign lesions, to detect hidden malignant tumors (staging) and to evaluate the response to anti-cancer chemotherapy1. However, inflammatory lesions (e.g., tuberculosis [TB], sarcoidosis and histoplasmosis) have also been reported to produce positive signals during 18F-FDG-PET scanning2. Thus, TB that manifests as well-circumscribed nodules or masses may be misdiagnosed as malignancy by 18F-FDG-PET3. In one report performed in TB-endemic area, nine of ten tuberculomas showed high glucose metabolism by 18F-FDG-PET scanning4.
South Korea is an intermediate TB-burden country with a continuously increasing incidence of malignancy. Thus, whole body PET scanning for cancer staging may detect asymptomatic lesions such as tuberculoma. To date, however, there have been no studies of 18F-FDG-PET scanning in patients with concomitant malignancy and tuberculoma except one case report5. We have therefore evaluated 18F-FDG-PET findings in patients with concomitant malignancy and tuberculoma. We also evaluated the effect of anti-TB treatment on 18F-FDG-PET results; this is especially important in patients with tuberculoma, since these lesions are difficult to monitor for response if radiographic size does not change.
The records of 12 patients with tuberculoma (manifested as mass or nodule) who underwent 18F-FDG-PET due to suspicion of malignancy or for metastasis work-up between January 2004 and December 2005 at Asan Medical Center (Seoul, South Korea) were retrieved retrospectively. TB lesions were confirmed bacteriologically or histologically in all subjects, and malignancies were confirmed by cytologic or histologic examination (n=11) or by angiographic findings (n=1, hepatoma). Clinical characteristics were analyzed by review of medical records. This study was approved by the Institutional Review Board of the Asan Medical Center.
All patients underwent the standard protocol used for 18F-FDG-PET scans at Asan Medical Center. Patients fasted for at least 6 hours before PET scan, and serum glucose concentrations were maintained below 120 mg/dL. After intravenous injection of about 550 MBq of 18F-FDG, patients received 10 mg of intravenous furosemide to accelerate renal 18F-FDG elimination. PET scans were performed using a dedicated PET scanner (ECAT HR+, SIEMENS) 60 minutes after tracer administration. Transmission scans were performed to provide attenuation correction with a 68Ge point source. The emission scan time was 6 min/bed and the transmission scan time was 4 min/bed position, with about five bed positions acquired from skull base to pelvis. PET scan data were reconstructed iteratively using an ordered subset expectation-maximization method with and without attenuation correction. 18F-FDG-PET images were reviewed by an experienced nuclear medicine physician on a dedicated workstation and compared with the results of chest computerized tomography (CT) or radiography. The maximum standardized uptake value (SUVmax) of each tumor and each TB lesion was determined.
The time-point of 18F-FDG-PET scans, performed at initial diagnosis of malignancy and tuberculoma (i.e., within 1 month after the start of anti-TB treatment), was designated as 'T0', and the time-point of follow-up 18F-FDG-PET scans, performed during or within 6 months after completion of anti-TB treatment, was designated as 'T1'.
Statistical analyses were performed using SPSS statistical software version 12.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±SD for continuous variables, and percentages for categorical variables. Paired numeric data were compared using the Mann-Whitney and Wilcoxon signed rank test. p-values < 0.05 were considered significant.
Of the 12 patients, 10 were male; their mean age was 67.2±7.9 years (range, 52~69 years). All 10 patients who underwent serologic tests for HIV infection showed negative results. Three patients (25.0%) had underlying systemic diseases (diabetes mellitus in one and chronic liver disease in two). Two patients had a history of previous anti-TB treatment and eight patients were present or former smokers.
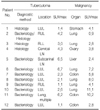
The sites of malignancies were the lung (n=6), colon (n=3), stomach (n=1), ovaries (n=1), and liver (n=1). Active TB was diagnosed bacteriologically (n=7) or histologically (n=5). Ten patients had pulmonary tuberculoma and the other two had TB lymphadenopathy (Table 1). Drug susceptibility was assessed in four patients, all of whom were pan-susceptible to first line anti-TB drugs.
The baseline SUVmax was higher in malignancy than tuberculoma, but the difference did not reach statistical significance (5.2±3.2 vs 3.5±2.0, p=0.147). In 4 patients, the SUVmax of the tuberculoma was higher than that of the malignancy (Table 1).
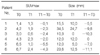
For their tumors, 11 patients were treated by surgical excision, with or without chemotherapy; the remaining patient refused treatment. All 12 patients completed anti-TB treatment successfully. The size of tuberculoma on CT decreased significantly after treatment, from 18.0±9.3 to 10.7±8.3 mm (p=0.043), but in one patient the size did not change (Table 2). Six patients underwent follow-up 18F-FDG-PET for evaluation of malignancy, with a mean interval of 298.0±180.5 days between T0 and T1. The SUVmax of all tuberculomas (including the tuberculoma that did not change in size) decreased significantly, from 3.5±2.0 at T0 to 1.6±0.87 at T1 (p=0.028) (Table 2) (Figure 1).
To our knowledge, this study is the first, except a case report, to compare the SUVmax of malignancy and tuberculoma in patients with both conditions. We found that SUVmax alone could not differentiate between malignancy and tuberculoma. In contrast, 18F-FDG PET was useful in monitoring the response to anti-TB treatment, especially in patients with tuberculomas that showed no radiographic changes in size after treatment.
Although to date, many 18F-FDG PET studies have analyzed whether SUVmax can differentiate between malignancy and TB6-9, none has enrolled subjects with concomitant malignancy and TB. While radiography can differentiate pulmonary TB from lung cancer lesions, the tuberculous lesions manifesting as tuberculomas are sometimes misdiagnosed as presumptive lung malignancy. Pulmonary tuberculomas are well-circumscribed nodules or masses in the lungs. Acid-fast bacilli can be identified in about half of these lesions, either by smear or by culture. In the remainder, TB is usually diagnosed microscopically and/or by a positive polymerase chain reaction for M. tuberculosis, as well as by the absence of other organisms such as Histoplasma.
Our study was performed in South Korea, a TB-endemic country; hence the concomitant presence of malignancy and tuberculoma in a patient is not a rare phenomenon. We found that SUVmax alone could not differentiate between tumor and tuberculoma. We also found that in 4 of our 12 patients (33.3%), the tuberculoma had higher SUVmax values than did the tumors. These finding suggest that, in TB-endemic areas, patients with pathologically confirmed malignant lesions and other hypermetabolic nodules should undergo biopsy and pathologic examination of the accompanying nodule to differentiate between malignant nodules and benign nodules such as tuberculomas.
Few studies to date have used 18F-FDG PET scanning to evaluate the therapeutic response of TB patients to anti-TB treatment5,10,11. Recently, in a study using murine TB model, serial 18F-FDG PET activity correlated with bactericidal activity of anti-TB treatment, showing the possibility of application of noninvasive imaging to monitor TB treatment response12. Although it is relatively easy to evaluate therapeutic responses in TB patients, using chest radiography and consecutive bacteriologic assays, some tuberculomas do not decrease in size or may even keep growing following anti-TB treatment13, making it difficult to evaluate therapeutic response. In a recent report, all 14 patients with TB or nontuberculous mycobacterial diseases showed decrement of SUVmax during or after anti-mycobacterial treatment11. Although follow-up 18F-FDG PET scans were performed to evaluate anti-malignancy treatment in our study, six patients underwent serial PET scans at T0 and T1, allowing us to evaluate the therapeutic response to anti-TB treatment by PET scanning. All six patients completed anti-TB treatment successfully, and the SUVmax of all 6 tuberculomas decreased significantly from baseline. This was especially important in one patient, who showed no change in tuberculoma size on CT, but showed a decrease in SUVmax, from 4.3 to 2.3. These findings suggest that 18F-FDG PET may be useful in monitoring the response to anti-TB treatment, especially in patients in whom it is difficult to otherwise evaluate treatment response.
Our study had the limitations inherent to retrospective studies. First, our sample size was small, because enrollment was restricted to patients with mass or nodular TB lesions (tuberculoma), although larger numbers of patients had concomitant malignancy and TB. The second limitation was associated with therapeutic response to anti-TB treatment. Although all patients underwent 18F-FDG PET before or within 1 month after the start of anti-TB treatment, follow-up 18F-FDG PET were not performed at same time of treatment completion because 18F-FDG PET is not a routine test for TB.
In conclusion, we found that SUVmax alone could not differentiate between malignancy and tuberculoma in patients with both conditions. We found, however, that 18F-FDG PET may be useful in monitoring response to anti-TB treatment, especially when treatment response cannot be evaluated by conventional radiologic methods such as simple chest radiography or CT. Further prospective studies in larger patient cohorts are needed to confirm these results.
Figures and Tables
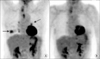
Figure 1
Serial PET scan results in a representative patient with concomitant lung cancer and tuberculoma. A (Baseline) and B (After treatment). Lung cancer (solid arrow) and tuberculoma (dotted arow) disappeared after treatment. The lung cancer was removed by lobectomy and the tuberculoma was treated with first-line anti-tuberculosis drugs.
References
1. Reske SN, Kotzerke J. FDG-PET for clinical use: results of the 3rd German Interdisciplinary Consensus Conference, "Onko-PET III", 21 July and 19 September 2000. Eur J Nucl Med. 2001. 28:1707–1723.
2. Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006. 7:57–69.
3. Sochocky S. Tuberculoma of the lung. Am Rev Tuberc. 1958. 78:403–410.
4. Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology. 2000. 216:117–121.
5. Hofmeyr A, Lau WF, Slavin MA. Mycobacterium tuberculosis infection in patients with cancer, the role of 18-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring treatment response. Tuberculosis (Edinb). 2007. 87:459–463.
6. Yen RF, Chen KC, Lee JM, Chang YC, Wang J, Cheng MF, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008. 35:1305–1315.
7. An YS, Sun JS, Park KJ, Hwang SC, Park KJ, Sheen SS, et al. Diagnostic performance of 18F-FDG PET/CT for lymph node staging in patients with operable non-small-cell lung cancer and inflammatory lung disease. Lung. 2008. 186:327–336.
8. Low SY, Eng P, Keng GH, Ng DC. Positron emission tomography with CT in the evaluation of non-small cell lung cancer in populations with a high prevalence of tuberculosis. Respirology. 2006. 1:84–89.
9. Hara T, Kosaka N, Suzuki T, Kudo K, Niino H. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest. 2003. 124:893–901.
10. Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008. 33:1–3.
11. Demura Y, Tsuchida T, Uesaka D, Umeda Y, Morikawa M, Ameshima S, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging. 2009. 36:632–639.
12. Davis SL, Nuermberger EL, Um PK, Vidal C, Jedynak B, Pomper MG, et al. Noninvasive pulmonary [18F]-2-fluoro-deoxy-D-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother. 2009. 53:4879–4884.
13. Lee HS, Oh JY, Lee JH, Yoo CG, Lee CT, Kim YW, et al. Response of pulmonary tuberculomas to anti-tuberculous treatment. Eur Respir J. 2004. 23:452–455.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download