Abstract
Background
The pathophysiologic mechanisms of early acute lung injury (ALI) differ according to the type of primary insult. It is important to differentiate between direct and indirect pathophysiologic pathways, and this may influence the approach to treatment strategies. NF-κB decoy oligodeoxynucleotide (ODN) is a useful tool for the blockade of the expression of NF-κB-dependent proinflammatory mediators and has been reported to be effective in indirect ALI. The purpose of this study was to investigate the effect of NF-κB decoy ODN in the lipopolysaccharide (LPS)-induced direct ALI model.
Methods
Five-week-old specific pathogen-free male BALB/c mice were used for the experiment. In the preliminary studies, tumor necrosis factor (TNF)-α, interleukine (IL)-6 and NF-κB activity peaked at 6 hours after LPS administration. Myeloperoxidase (MPO) activity and ALI score were highest at 36 and 48 hours, respectively. Therefore, it was decided to measure each parameter at the time of its highest level. The study mice were randomly divided into three experimental groups: (1) control group which was administered 50 µL of saline and treated with intratracheal administration of 200 µL DW containing only hemagglutinating virus of Japan (HVJ) vector (n=24); (2) LPS group in which LPS-induced ALI mice were treated with intratracheal administration of 200 µL DW containing only HVJ vector (n=24); (3) LPS+ODN group in which LPS-induced ALI mice were treated with intratracheal administration of 200 µL DW containing 160 µg of NF-κB decoy ODN and HVJ vector (n=24). Each group was subdivided into four experimental subgroups: (1) tissue subgroup for histopathological examination for ALI at 48 hours (n=6); (2) 6-hour bronchoalveolar lavage (BAL) subgroup for measurement of TNF-α and IL-6 in BAL fluid (BALF) (n=6); (3) 36-hour BAL subgroup for MPO activity assays in BALF (n=6); and (4) tissue homogenate subgroup for measurement of NF-κB activity in lung tissue homogenates at 6 hours (n=6).
Results
NF-κB decoy ODN treatment significantly decreased NF-κB activity in lung tissues. However, it failed to improve the parameters of LPS-induced direct ALI, including the concentrations of tumor necrosis factor-α and interleukin-6 in BALF, myeloperoxidase activity in BALF and histopathologic changes measured by the ALI score.
Acute respiratory distress syndrome (ARDS) has a more severe manifestation of acute lung injury (ALI) induced by a variety of predisposing conditions composed of direct and indirect insults1. Despite recent advances in the management of critically ill patients, the mortality rate of ARDS is persistently high2. Recently, the lung protective ventilation (LPV) strategy has been shown to improve the survival of ARDS patients by the prevention of ventilator-induced lung injury (VILI)3. The effectiveness of the LPV strategy may be limited because of severe spatial heterogeneity of lung involvement resulting in incomplete prevention of regional alveolar distension4. Therefore, alternative therapeutic strategies based on precise understanding of its pathophysiology are necessary to eliminate lung injury and improve the clinical consequences of ARDS patients.
During the pathogenesis of ALI and ARDS, the activation of transcriptional factor nuclear factor-κB (NF-κB) has been reported to be a pivotal mechanism by which the synthesis of multiple proteins contributes to inflammatory responses5-9. NF-κB is a ubiquitously expressed dimeric transcription factor involved in a wide range of biological processes that include inflammation, cell adhesion and cell survival10. Studies about the activation pathway of NF-κB led to the development of agents that block NF-κB transcriptional activity11-13. The decoy strategy14 is considered a useful tool for analyzing the blockade of the expression of a wide variety of NF-κB-dependent proinflammatory mediators15. NF-κB decoy oligodeoxynucleotide (ODN) is synthetic double stranded ODN with an NF-κB specific consensus sequence that binds to activated NF-κB. Thereby NF-κB decoy ODN competes for the binding of the activated NF-κB with consensus sequences in the promoter region of target genes16. The effect and role of NF-κB decoy ODN have been reported in various inflammatory models which were known to be related with NF-κB activation17-21. In particular, the NF-κB decoy ODN has been shown to be effective in the prevention of indirect ALI by intravenous lipopolysaccharide (LPS) administration22 and cecal ligation and puncture (CLP)-induced sepsis15.
The pathogenesis of ALI has been explained by the presence of a direct (primary or pulmonary) insult to the lung parenchyma such as pneumonia and aspiration of gastric contents, and/or indirect (secondary and extrapulmonary) insult, that results from an acute systemic inflammatory response such as sepsis and severe non-thoracic trauma with shock and multiple transfusions1,23. Various causes of ALI result in similar pathologies in the late stage24-27, but early pathophysiology, histopathology, respiratory mechanics, and response to therapeutic intervention may differ significantly according to the type of the primary insult28,29. According to the studies of experimental models of direct and indirect ALI, there are discernible differences in the expression of NF-κB dependent proinflammatory cytokines in bronchoalveolar lavage fluid (BALF)23, suggesting discrepancies in NF-κB activation between the two types of ALI.
The experimental methods were approved by the animal research committee of Korea University and the ethics committee of Korea University Medical Center. Five-week-old specific pathogen-free male BALB/c mice, each weighing 20 to 25 g, were used for the experiment. Each mouse was anesthetized by intraperitoneal injection of 65 mg/kg of pentobarbital sodium, and the trachea was exposed by mid-line cervical incision. LPS (E. coli O55 : B5, Sigma, St. Louis, MO, USA) 0.5 mg/kg/50 µL of saline was administered intratracheally with a microsyringe. To determine the time course of the parameters during ALI, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and myeloperoxidase (MPO) activity in bronchoalveolar lavage (BAL) fluid (BALF), histopathologic ALI score, and NF-κB activity in lung tissue homogenate were measured in BAL (n=6), tissue (n=6) and tissue homogenate (n=6) subgroups, respectively, at 6, 12, 24, 48, and 72 hours after LPS administration.
Under anesthesia, the mice of the tissue subgroup were tracheostomized and intubated. After rapid exsanguination by dissecting the abdominal aorta through an abdominal incision, the heart and lungs were removed en bloc through a midsternal incision. The lungs were immediately instilled with 4% paraformaldehyde through intubation at a hydrostatic pressure of 15 cmH2O and fixed in 4% paraformaldehyde for 48 hours. Paraffin blocks were prepared by dehydration of the lung tissues with ethanol and embedding in paraffin. For the mice in the BAL subgroup, after tracheostomy and intubation the thorax was opened following euthanasia by exsanguination, and three BAL procedures were performed, each with 1 mL of phosphate-buffered saline (PBS). The retrieval fluid was centrifuged (2,000×g at 4℃) for 10 minutes, and the supernatants were divided into aliquots and stored at -70℃ until analysis for TNF-α, IL-6 and MPO activity.
The posterior portions of the right lower lobe were sectioned at a thickness of 5 µm, placed on glass slides, and stained with hematoxylin-eosin. A pathologist, who was blinded to the protocol and experimental groups, examined the degree of lung injury and graded the specimens with an ALI score based on: (1) alveolar capillary congestion; (2) hemorrhage; (3) infiltration or aggregation of neutrophils in the airspace or the vessel wall; and (4) thickness of the alveolar wall/hyaline membrane formation. Each item was graded according to the following five-point scale: 0=minimal damage; 1=mild damage; 2=moderate damage; 3=severe damage; and 4=maximal damage. The degree of ALI was assessed by the sum of item scores from 0 to 16 in five randomly selected high-power fields (HPF, ×400). The average of the sum of each field score was compared among the groups30.
As an indicator of activated neutrophil accumulation, which is a major source of reactive oxygen species, the activity of MPO was determined directly in cell-free BALF according to the method described previously31, with minor modifications. Aliquots of 50 µL cell-free BALF were mixed in microtiter plates with 200 µL of O-dianisidine dihydrochloride (1.25 mg/mL in PBS) plus BSA (0.1% wt/vol) containing H2O2 (0.05%, or 0.4 mM). MPO activities are expressed as changes in absorbance at 450 nm. TNF-α and IL-6 in BALF were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). Nuclear proteins from tissue homogenate subgroup mice were prepared with a Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA) in accordance with the manufacturer's protocol. Activation of the NF-κB p65 subunit in 5 µg of nuclear extracts was measured using an NF-κB p65 ELISA-based transcription factor assay kit (TransAM™ NF-κB p65 Transcriptional Factor Assay Kit; Active Motif)32,33.
In the preliminary studies aimed to determine the time course of the parameters, TNF-α (Figure 1A), IL-6 (Figure 1B) and NF-κB activity (Figure 1E) peaked at 6 hours after LPS administration compared to the control group and other time points (p<0.05). MPO activity (Figure 1C) and ALI score (Figure 1D) were the highest at 36 and 48 hours, respectively (p<0.05). Therefore, it was decided to measure each parameter at the time of its highest level.
The following sequences of phosphorothioate double-stranded ODN against the NF-κB binding site were used in this study, which were the same as reported previously22,34: 5'-CCTTGAAGGGATTTCCCTCC-3' and 3'-GGAACTTCCCTAAAGGGAGG-5' (consensus sequences are underlined). For in vivo gene transfer, the hemagglutinating virus of Japan envelope vector system (HVJ Envelope Vector Kit GenomeOne-Neo; Ishihara Sangyo, Osaka, Japan) was used. This hemagglutinating virus of Japan envelope vector has been proven to be effective ODN delivery system both in vitro and in vivo15,35. To determine the effective treatment route and dose, NF-κB activity in lung tissue homogenate was measured after treatment with different routes and doses in the LPS-induced ALI model. The systemic route was tested with 200 µL of sterile distilled water (DW) containing different doses of NF-κB decoy ODN (4, 40, 80, and 160 µg/animal) infusion over 20 seconds through the tail vein, which was proven to be effective in sepsis-induced ALI models15,22, 1 hour after induction of LPS-induced ALI. At 6 hours after LPS administration, NF-κB activity showed decreasing trends with an increase in the ODN dose, but did not differ significantly from LPS-induced ALI control mice. However, in the intratracheal route experiment, NF-κB activity was significantly decreased with treatment of 160 µg ODN. Therefore, it was decided to treat with intratracheal administration of 160 µg of NF-κB decoy ODN.
The study mice were randomly divided into the following three experimental groups: (1) control group which was administered 50 µL of saline and treated with intratracheal administration of 200 µL DW containing only HVJ vector (n=24); (2) LPS group in which LPS-induced ALI mice were treated with intratracheal administration of 200 µL DW containing only HVJ vector (n=24); (3) LPS+ODN group in which LPS-induced ALI mice were treated with intratracheal administration of 200 µL DW containing 160 µg of NF-κB decoy ODN and HVJ vector (n=24). Each group was subdivided into four experimental subgroups: (1) tissue subgroup for histopathological examination for ALI at 48 hours (n=6); (2) 6-hour BAL subgroup for measurement of TNF-α and IL-6 in BALF (n=6); (3) 36-hour BAL subgroup for MPO activity assays in BALF (n=6); and (4) tissue homogenate subgroup for measurement of NF-κB activity in lung tissue homogenates at 6 hours (n=6).
All data are expressed as mean±standard deviation. Statistical analysis was performed using SPSS for Windows® (Release 11.0.1; SPSS Inc., Chicago, IL, USA). Intergroup differences were determined by non-parametric Mann-Whitney U and Kruskal-Wallis tests. Statistical significance was defined as p<0.05.
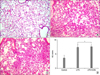
TNF-α and IL-6 were not detected in BALF of the control group. TNF-α concentration (Figure 2A) in the LPS group (2,445.7±103.72 pg/mL) was not different from that of the LPS+ODN group (2,529.67±32.86 pg/mL) (p=0.652). The concentration of IL-6 (Figure 2B) in the LPS and LPS+ODN groups (896.26±61.32 and 875.23±63.24 pg/mL, respectively) also did not show significant difference (p=0.723). Optical density (OD) of NF-κB activity in lung tissue homogenate (Figure 2C) was significantly different among the control, LPS, and LPS+ODN groups (0.043±0.07, 0.22±0.009 and 0.167±0.012 OD, respectively) (p=0.000 by Kruskal-Wallis test). The LPS+ODN group showed significantly lower NF-κB activity than the LPS group (p=0.004). MPO activity (Figure 2D) in the LPS+ODN group (0.183±0.114 OD) was not different from that of the LPS group (0.186±0.069 OD) (p=0.526). Histopathologic examination of the LPS group (Figure 3B) indicated high levels of ALI parameters, including intra-alveolar exudates, hyaline membrane formation, inflammatory cell infiltration, intra-alveolar hemorrhage, and interstitial edema. These findings of ALI were similar in the LPS+ODN group (Figure 3C). In the ALI score quantitative comparison (Figure 3D), the LPS+ODN group (12.1±1.2) showed an insignificant score in comparison to the LPS group (11.8±1.8) (p=0.453).
In this study, although intratracheal administration of NF-κB decoy ODN significantly decreased NF-κB activity in lung tissues, it failed to improve the parameters of LPS-induced direct ALI, including inflammatory cytokines, oxidant stress and histopathologic changes. These results are different from other studies that used indirect ALI models induced by intravenous injection of LPS22 and CLP15.
A vast majority of the results from the extensive animal studies demonstrated the complexity and multiplicity of the pathways in the pathologic processes of ALI, and they indicated that differences in the initial insult combined with underlying conditions can result in the activation of different inflammatory mechanisms29. Although various causes of ALI result in a uniform pathologic condition in the late stage, evidence indicates that the pathophysiology of early ALI may differ according to the type of primary insult23. Therefore, it is important to differentiate between direct and indirect pathophysiologic pathways, and this may influence the approach to treatment strategies, especially during the early phase. The acute phase of ALI is characterized by initiation and amplification of inflammatory response with a complex network of cytokines and other proinflammatory compounds36. Although functional and morphologic changes were similar, direct insult yielded more pronounced inflammatory responses independent of the cause; for example, pulmonary ALI showed a threefold increase in IL-8 and IL-10 in relation to extrapulmonary ALI, whereas IL-6 was two times greater in pulmonary ALI37, suggesting the importance of differentiation between direct and indirect pathophysiologic pathways.
NF-κB has been reported to be a critical transcription factor required for the maximal expression of many cytokines involved in the pathogenesis of ALI. Several studies on the NF-κB activation pathway led to the discovery of new agents capable of blocking NF-κB transcriptional activity11-13. The decoy strategy has recently been developed14 and is considered a useful tool for the blockade of the expression of a wide variety of NF-κB-dependent proinflammatory mediators. Inhibition of NF-κB activation with the use of a decoy ODN has been shown to be effective in myocardial infarction34, abdominal aortic aneurysm18, carotid artery intimal hyperplasia38, endotoxin-induced fatal liver failure19, and autoimmune myocarditis21 models. Also in the indirect murine ALI models using intravenous LPS injection15 and CLP22, this strategy has been effective in the inhibition of lung vascular permeability and sepsis-induced gene overexpression.
However, the present study with an LPS-induced direct ALI model showed that NF-κB decoy ODN had no effect on concentrations of TNF-α and IL-6, MPO activity and histopathologic lung injury in spite of a statistically significant decrease of NF-κB activity in lung tissues. The most plausible reason for these results that differ with those of indirect ALI models is the difference of NF-κB decoy ODN effectiveness depending on the time course of NF-κB activation. In the LPS- and CLP-induced indirect ALI models, it was reported that NF-κB activity was markedly increased in the lung tissues from 10 hours after insult15,22, but intratracheal LPS administration showed peak NF-κB activity at 6 hours and rapid decline down to the level of the control at 12 hours, indicating earlier activation of NF-κB by direct insult. Another issue is the action time of NF-κB decoy ODN. Although precise time courses of the effectiveness of NF-κB decoy ODN in different models have not been elucidated, Matsuda et al. reported that the tail vein injection of 80 µg NF-κB decoy ODN produced a constant reduction >70% in the activation of NF-κB in lung tissues at 9 hours after injection in LPS- or CLP-induced indirect ALI models15,22. However, in our preliminary studies for the determination of treatment route, tail vein injection of NF-κB decoy ODN up to 160 µg had no significant effect in the reduction of NF-κB activity in lung tissues in the LPS-induced direct ALI model. The absence of the effect of systemic treatment with NF-κB decoy ODN in the direct ALI model is suspected to be due to the different compartmentalization of inflammatory response39 compared with indirect ALI models and the possibility of inactivation of HVJ envelope by adsorption to blood cells (by manufacture's guide). Therefore, the intratracheal treatment route was adopted. In spite of the significant decrease of NF-κB activity by intratracheal treatment with 160 µg of NF-κB decoy ODN, the degree of inhibition was about 24% compared with the LPS group which was considerably lower than those of the studies using indirect ALI models. These results may be caused by the fact that in the direct ALI model acute inflammatory response is earlier and more intense than the indirect models; also, the maximal action time of NF-κB decoy ODN is later than the time of NF-κB activation in the direct ALI model. In order to answer this question, proper studies about the time course of the effectiveness of NF-κB decoy ODN and studies with more than 160 µg ODN would be necessary. Taking into consideration the maximal effect time of 9 hours after ODN injection in direct ALI models, pretreatment with ODN prior to LPS administration may be essential to be effective in the inhibition of NF-κB activation in the direct ALI model which shows an earlier NF-κB activation than the indirect model. However, pretreatment strategy has no clinical relevance in investigating the treatment modalities for ALI and ARDS patients. Studies using more than 160 µg of the ODN in intratracheal treatment are almost impossible because of the solubility limitation of ODN and the vector system and a possibility of drowning the mice due to an increase in the administration volume of treatment solution. There were several limitations in this study. First, the experiments using a group treated with scrambled decoy ODN were absent. Because treatment with NF-κB decoy ODN did not show any effect on the parameters of ALI, the experiments with scrambled decoy ODN were not performed. Second, the experiment with control group treated by NF-κB decoy ODN without HVJ vector system was omitted. Therefore, possible detrimental effects of NF-κB decoy ODN such as an insult for ALI were not examined. Third, treatment with NF-κB decoy ODN using intratracheal administration route could not guarantee even distribution of the ODN. Although intratracheal treatment with 160 µg of NF-κB decoy ODN decreased significantly NF-κB activity, limitation of inhibitory effect due to uneven distribution of the ODN could not be ruled out.
In order to discover new therapeutic strategies for ALI and ARDS, it is essential to detail cellular and molecular mechanisms that modulate inflammation and protect the lung from biomechanical stress injury40. According to the results of this study, NF-κB decoy ODN, which has been proven to be effective in indirect models, had no effect in the direct ALI model.
Figures and Tables
Figure 1
The time course of acute lung injury (ALI) parameters. The concentrations of tumor necrosis factor (TNF)-α (A) and interleukin (IL)-6 (B) in bronchoalveolar lavage fluid (BALF) and nuclear factor (NF)-κB activity (E) in lung tissue homogenates showed peaks at 6 hours after lipopolysaccharide (LPS) administration. Myeloperoxidase (MPO) activity (C) and ALI score (D) were highest at 36 and 48 hours, respectively (*p<0.05, compared with the control and other time points).

Figure 2
The effects of nuclear factor (NF)-κB decoy oligodeoxynucleotide (ODN). The concentrations of tumor necrosis factor (TNF)-α (A) and interleukin (IL)-6 (B) and myeloperoxidase (MPO) activity (D) between the lipopolysaccharide (LPS) and LPS+ODN groups were not significantly different. NF-κB activity (C) in the lung tissue homogenates of the LPS+ODN group was significantly lower than the LPS group (*p=0.004, †p>0.05).

Figure 3
Histopathologic examination (hematoxylin-eosin, ×100) and quantification by acute lung injury (ALI) score. The lipopolysaccharide (LPS) group (B) showed high levels of intra-alveolar exudates, hyaline membrane formation, inflammatory cell infiltration, intra-alveolar hemorrhage, and interstitial edema. These findings were similar with the LPS+oligodeoxynucleotide (ODN) group (C). The ALI score (D) of the LPS+ODN group was not different from the LPS group (*p=0.453).
References
1. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.
2. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005. 353:1685–1693.
3. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. 342:1301–1308.
4. Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001. 164:1701–1711.
5. Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage tumor necrosis factor activity and alters NF-kappaB expression. Am J Physiol. 1999. 276:L909–L916.
6. Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000. 13:85–91.
7. Pepperl S, Dörger M, Ringel F, Kupatt C, Krombach F. Hyperoxia upregulates the NO pathway in alveolar macrophages in vitro: role of AP-1 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L905–L913.
8. Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, et al. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998. 275:L1040–L1050.
9. Shenkar R, Yum HK, Arcaroli J, Kupfner J, Abraham E. Interactions between CBP, NF-kappaB, and CREB in the lungs after hemorrhage and endotoxemia. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L418–L426.
10. Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999. 274:27339–27342.
11. Nathens AB, Bitar R, Davreux C, Bujard M, Marshall JC, Dackiw AP, et al. Pyrrolidine dithiocarbamate attenuates endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 1997. 17:608–616.
12. Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997. 272:21096–21103.
13. Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002. 61:953–963.
14. Bielinska A, Shivdasani RA, Zhang LQ, Nabel GJ. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990. 250:997–1000.
15. Matsuda N, Hattori Y, Jesmin S, Gando S. Nuclear factor-kappaB decoy oligodeoxynucleotides prevent acute lung injury in mice with cecal ligation and puncture-induced sepsis. Mol Pharmacol. 2005. 67:1018–1025.
16. Morishita R, Higaki J, Tomita N, Ogihara T. Application of transcription factor "decoy" strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res. 1998. 82:1023–1028.
17. Igwe OJ. Modulation of peripheral inflammation in sensory ganglia by nuclear factor (kappa)B decoy oligodeoxynucleotide: involvement of SRC kinase pathway. Neurosci Lett. 2005. 381:114–119.
18. Nakashima H, Aoki M, Miyake T, Kawasaki T, Iwai M, Jo N, et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor kappaB and ets transcription factors. Circulation. 2004. 109:132–138.
19. Ogushi I, Iimuro Y, Seki E, Son G, Hirano T, Hada T, et al. Nuclear factor kappa B decoy oligodeoxynucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology. 2003. 38:335–344.
20. Xu MQ, Shuai XR, Yan ML, Zhang MM, Yan LN. Nuclear factor-kappaB decoy oligodeoxynucleotides attenuates ischemia/reperfusion injury in rat liver graft. World J Gastroenterol. 2005. 11:6960–6967.
21. Yokoseki O, Suzuki J, Kitabayashi H, Watanabe N, Wada Y, Aoki M, et al. cis Element decoy against nuclear factor-kappaB attenuates development of experimental autoimmune myocarditis in rats. Circ Res. 2001. 89:899–906.
22. Matsuda N, Hattori Y, Takahashi Y, Nishihira J, Jesmin S, Kobayashi M, et al. Therapeutic effect of in vivo transfection of transcription factor decoy to NF-kappaB on septic lung in mice. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1248–L1255.
23. Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003. 42:48s–56s.
24. Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977. 116:589–615.
25. Blaisdell FW. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974. 108:44–49.
26. Lamy ML, Fallat RJ, Koeniger EL, Dietrich HP, Kamm B, Hill JD. Pathophysiology of adult respiratory distress syndrome. Acta Anaesthesiol Belg. 1975. 23:Suppl. 64–77.
27. Nash G, Foley FD, Langlinais PC. Pulmonary interstitial edema and hyaline membranes in adult burn patients. Electron microscopic observations. Hum Pathol. 1974. 5:149–160.
28. Callister ME, Evans TW. Pulmonary versus extrapulmonary acute respiratory distress syndrome: different diseases or just a useful concept? Curr Opin Crit Care. 2002. 8:21–25.
29. Rocco PR, Zin WA. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005. 11:10–17.
30. Kim JH, Suk MH, Yoon DW, Lee SH, Hur GY, Jung KH, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L580–L587.
31. Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996. 270:L836–L845.
32. Harada K, Ohira S, Isse K, Ozaki S, Zen Y, Sato Y, et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest. 2003. 83:1657–1667.
33. Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, et al. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001. 29:E21.
34. Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997. 3:894–899.
35. Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther. 2002. 6:219–226.
36. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.
37. Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005. 98:1777–1783.
38. Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element 'decoy' of nuclear factor-kappaB binding site as a novel molecular strategy. Gene Ther. 2001. 8:1635–1642.
39. Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006. 12:151–170.
40. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003. 167:1027–1035.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download