Abstract
An intramedullary spinal cord metastasis (ISCM) rarely develops in systemic cancer but is indicative of a poor prognosis. A 56-year-old man was admitted due to weakness of the lower extremities. He had received radiotherapy 3 months prior for a brain metastasis that had developed 1 year after achieving a complete response from chemotherapy for extended stage small cell lung cancer. Although the brain lesion had improved partially, ISCM from the cervical to lumbar-sacral spinal cords, which was accompanied by a leptomeningeal dissemination, was diagnosed based on magnetic resonance imaging of the spine and cerebrospinal fluid cytology. Finally, he died of sudden cardiac arrest during treatment. This is the first case of ISCM involving the whole spinal segments. Physicians should be aware of the subsequent development of ISCM in lung cancer patients with a previously known brain metastasis who present with new neurological symptoms.
Intramedullary spinal cord metastasis (ISCM) is an unusual occurrence in systemic cancer with a frequency of 0.9~2.1% in all autopsy cases of cancer1 and 8.5% of central nervous system metastasis2. Lung cancer, mostly small cell carcinoma, accounts for almost half of ISCM3 and others such as cancers of breast, colon, kidney and melanoma also can lead to this rare form of metastasis4. In most cases, one segment of the spinal cord is usually involved although metastasis to two adjacent segments can rarely occur. Herein, we report the first case of ISCM involving the whole spinal segments and discuss the associated metastatic route.
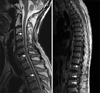
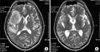
A 56-year-old man was diagnosed to have extended stage small cell lung cancer with malignant effusion 3 years prior to admission. There was no distant metastasis. He received 6 cycles of chemotherapy with etoposide (100 mg/m2, day 1~3) and cisplatin (80 mg/m2, day 1), which led to complete response. Follow-up chest CT, CBC and blood chemistry was done regularly every two months on an outpatient basis. However, headache and dizziness developed almost 1 year after chemotherapy. Brain MRI showed a 6 cm-sized mass on right frontal and temporal lobe with peritumoral edema. There was no aggravating lesion in other sites including lung, pleura and bone. Brain radiotherapy of 3,000 cGy was done which led to symptomatic improvement. 3 months later, he developed weakness of both lower extremities. The previous metastatic brain lesion showed further improvement on follow-up brain MRI (Figure 1). However, spine MRI revealed multiple enhancing nodules in the entire spinal cord from cervical to lumbar-sacral segments (Figure 2). Tumor cells were also found on cytologic examination of CSF suggesting accompanied leptomeningeal seeding (Figure 3). There was no abnormal finding in the leptomeninges on both brain and spine MRI. Although palliative radiotherapy to the spinal cord and intrathecal chemotherapy with methotrexate (15 mg/m2, twice/week) and hydrocortisone (15 mg/m2, twice/week) was given, his condition deteriorated and died of sudden cardiac arrest.
The most common site of ISCM among segments of the spinal cord vary according to reports from cervical cord to lumbar-sacral region4,5. Nonetheless, it can be recognized that mostly one segment of the cord is usually involved although metastasis to two adjacent segments could rarely occur. To our knowledge, this is the first case of ISCM involving the whole spinal segments.
ISCMs typically develop through arterial dissemination although direct invasion from spinal roots or leptomeninges or venous spread can also result in its developement6. Direct invasion can occur when tumor cells infiltrate the Virchow-Robin spaces of the penetrating vessels of the spinal cord and subsequently penetrate the pial membrane and invade the spinal cord parenchyma7. Meanwhile, venous spread of tumor cells develops through Batson's plexus extending from the pelvis to the cranial venous sinuses, which enables retrograde transportation to the spinal cord8.
Coexistence of brain metastasis and ISCM have been found more frequently than ISCM with leptomeningeal dissemination suggesting the vascular route as the more favorable mode of spread5. However, there seems to be a strong association between leptomeningeal seeding and ISCM, particularly in small cell lung cancer considering the fact that 15 out of 28 patients with ISCM from small cell lung cancer had combined carcinomatous meningitis9. Malignant cells were detected from CSF cytology of our patient indicating simultaneous leptomeningeal metastasis although there was no radiological evidence such as thickening or enhancement of membranes on MRI.
Because three forms of central nervous system metastasis were finally noted in our patient, it seems very difficult to define the causal relationship between them. As for the mechanism of metastasis, it seems probable that the CSF route contributed more in our patient because of involvement of the whole spinal segments.
When our patient presented a newly developed neurologic symptom, weakness of the lower extremities, we were initially concerned about aggravation of the metastatic brain lesion in spite of radiotherapy. As the brain MRI revealed improvement of the brain metastasis without any new lesion, the pathology in leptomeninges or spinal cord was suspected prompting us to study the CSF and obtain a spine MRI. Lower motor neuron weakness is the most common among all cerebral, cranial nerve and spinal symptoms in leptomeningeal metastasis10. It is also one of most prevalent symptoms in ISCM along with pain and sensory loss4,5. Therefore, studies to rule out these two possibilities seem necessary to differentiate the cause of weakness of the lower extremities in the aspect that the symptoms can be caused by either of them or even both concomitantly.
Figures and Tables
Figure 1
Multiple enhancing nodules with high signal intensity (arrows) on T2-weighted image of MRI were found over entire spinal cords from cervical spine to lumbar-sacral region.
References
1. Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia: a prospective autopsy study. Cancer. 1963. 16:781–787.
2. Moffie D, Stefanko SZ. Intramedullary metastasis. Clin Neurol Neurosurg. 1980. 82:199–202.
3. Grem JL, Burgess J, Trump DL. Clinical features and natural history of intramedullary spinal cord metastasis. Cancer. 1985. 56:2305–2314.
4. Okamoto H, Shinkai T, Matsuno Y, Saijo N. Intradural parenchymal involvement in the spinal subarachnoid space associated with primary lung cancer. Cancer. 1993. 72:2583–2588.
5. Potti A, Abdel-Raheem M, Levitt R, Schell DA, Mehdi SA. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer. 2001. 31:319–323.
6. Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis: a clinicopathological study of 13 cases. J Neurosurg. 1985. 62:227–233.
7. Kalayci M, Cağavi F, Gül S, Yenidünya S, Açikgöz B. Intramedullary spinal cord metastases: diagnosis and treatment - an illustrated review. Acta Neurochir (Wien). 2004. 146:1347–1354. discussion 1354.
8. Grem JL, Burgess J, Trump DL. Clinical features and natural history of intramedullary spinal cord metastasis. Cancer. 1985. 56:2305–2314.
9. Weissman DE, Grossman SA. Simultaneous leptomeningeal and intramedullary spinal metastases in small cell lung carcinoma. Med Pediatr Oncol. 1986. 14:54–56.
10. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982. 49:759–772.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download