Abstract
Background
Dust clouds blown by the wind from the arid deserts of Mongolia and Northeast China are known as Asian dust storms. Ambient particulate matter with a diameter <10 µm (PM10) is associated with the exacerbation of respiratory diseases and increased mortality of heart and lung disease patients. The fibrotic effects of PM10 of Asian dust to pulmonary fibroblast cells are unknown. This study examined the production of reactive oxygen species (ROS), TGF-β, NF-κB, PDGF-α and Fibronectin in fibroblasts exposed to Asian dust particles.
Methods
Air samples were collected using a high volume air sampler (Sibata model HV500F) with an air flow of 500 L/min for at least 6 hours. The MRC-5 cells were exposed to 0, 50 and 100 µg/mL of PM10 for 24 hours. ROS was detected by measuring the level of oxidized DCF using FACS. TGF-β, NF-κB, PDGF-α and fibronectin were detected by western blotting.
Results
There was no increase in the ROS, TGF-β and PDGF-α levels in the MRC-5 cells exposed to PM10. The NF-κB level was higher in the MRC-5 cells exposed to 50 and 100 µg/mL of PM10 for 24 hours. The fibronectin level in the MRC-5 cells after 24 hours incubation with 50 µg/mL PM10 was significantly higher than the control group (PM10 50 µg/mL 113.27±8.65 of control, p=0.005).
Figures and Tables
Figure 1
Measured Reactive oxygen species (ROS) by FACS in MRC-5 cells after 24 hours incubation with PM10. MRC-5 cells were exposed to 50 and 100 µg/mL of ambient particulate matter with a diameter of less than 10 µm for 24 hours. Results shown in the box graph are measured ROS values by FACS. The measured ROS in MRC-5 cells after 24 hours incubation with PM10 were not increased (PM10 0 µg/mL 113.01±83.4, PM10 50 µg/mL 92.48±70.46, p=0.674, PM10 100 µg/mL 106.80±88.73, p=0.916).

Figure 2
Confocal microscopic findings in MRC-5 cells after 24 hours incubation with PM10. The fluorogenic probe 2'7'-dichlordihydrofluorescin diacetate (DCF-DA) in the cell was oxidized and detected as green color. The oxidized DCF-DA was not increased in MRC-5 cells after 24 hours incubation with PM10.

Figure 3
TGF-β in MRC-5 cells after 24 hours incubation with PM10. TGF-β was detected by western blotting. The density of band was measured by densitometry. Results shown in the bar graph are the percentage from control values. TGF-β in MRC-5 cells after 24 hours incubation with PM10 was not increased compared with control group (PM10 50 µg/mL 101.43±6.99 of control, p=1.000, PM10 100 µg/mL 97.91±26.40 of control, p=1.000).
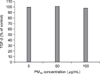
Figure 4
NF-κB in MRC-5 cells after 24 hours incubation with PM10. MRC-5 cells were exposed to 50 and 100 µg/mL of ambient particulate matter with a diameter of less than 10 µm for 24 hours. NF-κB was detected by western blotting.

Figure 5
PDGF-α in MRC-5 cells after 24 hours incubation with PM10. PDGF-α was detected by western blotting. The density of band was measured by densitometry. Results shown in the bar graph are the percentage from control values. Results shown in the bar graph are the percentage from control values (*p<0.05). PDGF-α in MRC-5 cells after 24 hours incubation with 50 µg/mL PM10 was not significantly increased compared with control group (PM10 50 µg/mL 101.94±8.13 of control, p=0.487). PDGF-α in MRC-5 cells with 100 µg/mL PM10 was signifi cantly decreased compared with control group (PM10 100 µg/mL 97.50±1.81 of control, p=0.037).
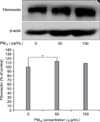
Figure 6
Fibronectin and β-actin in MRC-5 cells after 24 hours incubation with PM10. MRC-5 cells were exposed to 50 and 100 µg/mL of ambient particulate matter with a diameter of less than 10 µm for 24 hours. Fibronectin was detected by western blotting. The density of band was measured by densitometry. Results shown in the bar graph are the percentage from control values (*p<0.05). Fibronectin in MRC-5 cells after 24 hours incubation with 50 µg/mL PM10 was significantly increased compared with control group (PM10 50 µg/mL 113.27±8.65 of control, p=0.005). Fibronectin in MRC-5 cells with 100 µg/mL PM10 was not increased compared with control group (PM10 100 µg/mL 107.82±11.91 of control, p=0.577).

References
1. Bell ML, Davis DL. Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution. Environ Health Perspect. 2001. 109:Suppl 3. 389–394.
2. Donaldson K, Stone V, Clouter A, Renwick L, MacNee W. Ultrafine particles. Occup Environ Med. 2001. 58:211–216.
3. Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, et al. Effects of ambient particulate matter (PM10) on peak expiratory flow and respiratory symptoms in subjects with bronchial asthma during yellow sand period. Tuberc Respir Dis. 2003. 55:570–578.
4. Donaldson K, Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003. 39:405–410.
5. Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996. 141:637–648.
6. Kim JH, Jeon HK, Kim MK, Kyung SY, An CH, Lee SP, et al. Particulate matter from Asian dust storms induces the expression of proinflammatory cytokine in A549 epithelial cells. Tuberc Respir Dis. 2006. 60:663–672.
7. Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006. 3:413–417.
8. Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006. 3:350–356.
9. Hwang HJ, Kang SJ, Kang SE, Park YM, Kim HK, Roh CU. Single particle characterization of aerosol samples collected during Asian dust storm events and non-Asian dust period in Incheon. Proceedings of the 2007 Environmental Societies Joint Conference. 2007. 2007 May 2-4; Busan, Korea. Seoul: Korean Society for Atmospheric Environment;179–182.
10. Baek KW, Chung JD. Study on the yellow sandy dust phenomena in Korean peninsula and chemical compositions in fine particles at background sites of Korea. Korean J Sanit. 2004. 19:9–18.
11. Park KS, Kim YJ, Yoon JY, Kyung SY, An CH, Lee SP, et al. Particulate matter 10 from Asian dust storms induces the expression of reactive oxygen species, NF-kappaB, TGF-beta and fibronectin in WI-26 VA4 epithelial cells. Tuberc Respir Dis. 2008. 65:504–511.
12. Jiang Z, Seo JY, Ha H, Lee EA, Kim YS, Han DC, et al. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003. 309:961–966.
13. Rennard SI, Bitterman PB, Crystal RG. Response of the lower respiratory tract to injury. Mechanisms of repair of the parenchymal cells of the alveolar wall. Chest. 1983. 84:735–739.
14. Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, et al. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000. 165:2190–2197.
15. Churg A, Brauer M, del carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003. 111:714–718.
16. Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000. 23:182–187.
17. Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am J Respir Cell Mol Biol. 1998. 19:98–106.
18. Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000. 165:1013–1021.
19. Jiménez LA, Thompson J, Brown DA, Rahman I, Antonicelli F, Duffin R, et al. Activation of NF-kappaB by PM10 occurs via an iron-mediated mechanism in the absence of IkappaB degradation. Toxicol Appl Pharmacol. 2000. 166:101–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download