Abstract
Background
The methacholine bronchial provocation test is a useful tool for evaluating asthma in patients with normal or near normal baseline lung function. However, the sensitivity of this test is 82~92% at most. The purpose of this study is to evaluate the clinical usefulness of FEF25-75% in identification of airway hyperresponsiveness in patients with suspected asthmatic symptoms.
Methods
One hundred twenty-five patients who experienced cough and wheezing within one week prior to their visiting the clinic were enrolled.
Results
Sixty-four subjects showed no significant reduction of FEV1 or FEF25-75% on the methacholine bronchial provocation test (Group I). In 24 patients, FEF25-75% fell more than 20% from baseline without a 20% fall of FEV1 during methacholine challenge (Group II). All patients who had more than 20% fall of FEV1 (n=37) also showed more than 20% of reduction in FEF25-75% (Group III). Baseline FEV1/FVC (%) and FEF25-75% (L) were higher in group II than group III (81.51±1.56% vs. 75.02±1.60%, p<0.001, 3.25±0.21 L vs. 2.45±0.21 L, p=0.013, respectively). Group II had greater reductions of both FEV1 and FEF25-75% than group I at 25 mg/mL of methacholine (p<0.001). The provocative concentration of methacholine causing a 20% fall in FEF25-75% in group II was about three-fold higher than that in group III.
Asthma is a chronic airway inflammatory disease with a growing global prevalence. Wheezing, breathlessness, and cough are the major symptoms of asthma, but patients complain of variety of combinations of these symptoms. A combination of wheezing and dyspnea has the greatest positive predictive value for the diagnosis of asthma1. The bronchial provocation test is an important diagnostic tool to identify airway hyperresponsiveness in asthma2. Several textbooks indicate that negative result in the methacholine provocation test rules out asthma as a cause of chronic cough because the bronchial provocation test has very high sensitivity and negative predictive value3. However, in real practice, we frequently fail to prove the presence of airway hyperresponsiveness by the methacholine provocation test in patients with typical asthmatic symptoms. The positive rates of methacholine provocation test were 79.5% in physician-diagnosed asthma and only 37.7% in chronic cough with wheezing in Korea4,5. Traditionally, the methacholine provocation test uses the 'Forced Expiratory Volume in 1 second (FEV1)' to identify bronchial hyperresponsiveness. The 'Forced Expiratory Flow 25-75% (FEF25-75%)' is a more sensitive indicator for the obstruction of small airways than FEV1, but has lower reproducibility than FEV1. However, the insufficient sensitivity of the methacholine provocation test based on FEV1 necessitates the development of a more sensitive indicator. Thus we evaluated the role of a provocative concentration of methacholine causing a 20% fall in FEF25-75% (FEF25-75%-PC20) compared with PC20 in FEV1 (FEV1-PC20) to identify airway hyperresponsiveness in patients with suspected asthmatic symptoms.
Patients with cough and wheezing were recruited for this retrospective study. Eligible patients' records were acquired from the chronic cough database of Hallym University Sacred Heart Hospital between October, 2004 and July, 2007. Inclusion criteria were the followings: age over 15, current or a recent wheezing experience within one week prior to visiting the cough clinic, and a tolerable physical condition for the methacholine provocation test. Suspicious COPD, structural lung disease, tuberculosis, heart failure, and pulmonary edema were excluded. Smoking status was not considered in the selection criteria. After careful medical history taking and physical exams, chest and paranasal sinus view x-ray, differential cell count in induced sputum, and the methacholine bronchial provocation test were performed to identify the cause of cough and wheezing. The study design was approved by the institutional ethics committee.
The methacholine bronchial provocation test was performed as described by Chai et al6. After the baseline pulmonary function, methacholine aerosol at 0.625, 1.25, 2.5, 12.5, and 25 mg/mL was delivered with a compressor (Pulmo-Aide 5650D, DeVibliss Health Care, Somerset, PA, USA) and an aerosolizer (DeVibliss 646, DeVibliss Health Care). The patients inhaled the aerosol quantified by KoKo-MSM analog micro-dosimeter (nSpire Health, Inc. Longmont, CO, USA) slowly for five seconds and then held their breath for five seconds. The patients repeated this procedure 5 times, and then FEV1 was measured repeatedly after 30 and 90 seconds. The highest FEV1 was included in the analysis7.
The methacholine challenge test was stopped when FEV1 had fallen more than 20% from baseline or the final methacholine concentration of 25 mg/mL was applied. PC20 was calculated by linear interpolation regarding FEV1 fall (FEV1-PC20) and FEF25-75% fall (FEF25-75%-PC20). Patients were classified as positive responders when the PC20 was lower than 25 mg/mL. The patients were grouped according to methacholine provocation test: Group I, patients with both FEF25-75%-PC20 and FEV1-PC20 >25 mg/mL; Group II, patients with FEF25-75%-PC20 ≤25 mg/mL and FEV1-PC20 >25 mg/mL; Group III, both FEF25-75%-PC20 and FEV1-PC20 ≤25 mg/mL.
After measuring the baseline FEV1, the patients gargled with water and wiped their mouths with dry gauze. Sputum was induced with 15 mL of 4.5% hypertonic saline aerosol inhalation for five minutes. Afterwards the patients rinsed their mouth with water, and then expectorate sputum in a 50 mL Falcon tube. This procedure was repeated three to four times. The collected sputum was immediately treated with an equal volume of 0.15% (0.01 M) dithiothreitol dissolved in PBS and rocked for 30 minutes. Then it was filtered through a 100 µm mesh strainer and centrifuged at 2,000 rpm for five minutes at 4℃. The cell pellet was resuspended with 5 mL of PBS for differential cell count after careful removal of the supernatant. Cytospins were Wright-stained and differential cell counting of macrophages, neutrophils, lymphocytes, and eosinophils was done for more than 300 non-squamous cells. According to eosinophil percentages, patients were divided into two groups: increased eosinophil count (sputum eosinophilia) ≥3%, or normal eosinophil count <3%.
Independent samples t-tests were used to compare continuous variables from two groups. One-way ANOVA was used to compare three groups. Categorical variables were analyzed by chi-square test. Trends were analyzed by chi-square for linear trend. p-value <0.05 was considered significant.
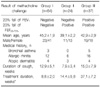
A total of 125 patients were enrolled for the analysis. Fifty-three patients were male and 72 were female. Sixty-four patients were classified in group I, 24 patients in group II, and 37 patients in group III according to the methacholine provocation test (Table 1). The mean age was 43.4±1.4 years and there was no difference among the groups. Group II was younger than the others, but not significantly. Eight (6.4%) of them had a medical history of bronchial asthma, 34 (27.2%) had allergic rhinitis, and 11 (8.8%) had previous atopic dermatitis. The duration of cough was 12.9±5.1 weeks in group I, 7.9±3.4 weeks in group II, and 15.0±7.9 weeks in group III, but the differences were not statistically significant. The treatment duration in the outpatient clinic was 8.8±2.0 weeks in group I, 14.4±5.8 weeks in group II, and 37.1±7.2 weeks in group III (p<0.001).
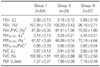
Group III showed the lowest mean values in all the parameters related with airway obstruction, such as FEV1, FEF25-75%, FEV1/FVC, and FEF25-75%/FVC (Table 2). Group II showed a greater reduction of FEV1 and FEF25-75% at the highest methacholine concentration of 25 mg/mL compared with group I. The FEV1 changes for group I and group II at 25 mg/mL were 6.6±0.6% vs. 12.7±0.7%, respectively (p<0.001). The FEF25-75% falls of group I and group II at 25 mg/mL were 1.4±1.8% vs. 28.4±1.7%, respectively (p<0.001) (Figure 1A). The FEF25-75%-PC20 of group II was about three-fold higher than that of group III (20.5±1.5 mg/mL vs. 7.4±1.0 mg/mL, respectively, p<0.001) (Figure 1B). These findings suggest that the bronchial hyperresponsiveness increased in group I, II, and III.
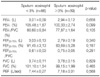
Sputum eosinophilia was present in 39 (31.2%) patients. The proportion of patients with sputum eosinophilia significantly increased with a rank order for group I (20.3%)>group II (29.2%)>group III (51.4%) (p=0.005). All parameters of airway obstruction in the pulmonary function test were lower in patients with sputum eosinophilia, but not significantly (Table 3). The methacholine provocation test was positive more often in patients with sputum eosinophilia. For example, in group III positive methacholine provocation tests for FEV1 with and without sputum eosinophilia were 48.7% vs. 20.9%, respectively (p=0.002). Positive methacholine tests for FEF25-75% in group II and III patients with and without sputum eosinophilia were 40.7% vs. 66.7%, respectively (p=0.007) (Figure 2A). Mean PC20 based on either FEV1 or FEF25-75% were significantly lower in patients with sputum eosinophilia. The FEV1-PC20 in group III patients with or without sputum eosinophilia was insignificant (9.9±1.8 mg/mL vs. 13.4±2.0 mg/mL respectively, p=0.203), but FEF25-75%-PC20 in group II and III patients with sputum eosinophilia was significantly lower than in patients without sputum eosinophilia (9.3±1.7 mg/mL vs. 14.4±1.6 mg/mL respectively, p=0.032) (Figure 2B). Twenty-two patients with sputum eosinophilia and 33 patients without sputum eosinophilia underwent bronchodilator response test. The positive rate of bronchodilator response (≥12% and ≥200 mL from baseline in FEV1) was higher in patients with sputum eosinophilia than in the patients without sputum eosinophilia (22.72% vs. 6.06%, p=0.027).
Bronchial asthma is characterized by variable airway obstruction due to constriction and edema of bronchus; therefore, a pulmonary function test with bronchodilators is used as an objective tool to prove the airway reversibility in symptomatic asthmatics. If lung function is normal or near normal at rest, non-specific bronchial provocation test can be done to confirm the presence of airway hyperresponsiveness1. Methacholine is a chemically stable acetylcholine-like substance that constricts the bronchial smooth muscle directly by cholinergic stimulation. Although methacholine is one of the most widely used non-specific bronchial stimulants, there is no unanimously adopted method for the methacholine bronchial provocation test. On inhaling methacholine, two different methods are frequently used, the 5-breath technique and tidal volume method7. The cutoff value of PC20 for positive response varies from 8 to 16 mg/mL. Despite the reported good test sensitivity, false negative can also present2. For example, extremely trained athletes, patients on bronchodilators or anticholinergic drugs, and patients without current symptoms or exposure allergen can show false negatives. Full inspiration up to total lung capacity (TLC) has a protective effect on bronchial constriction; therefore TLC inhalation with the 5-breath technique can produce false negatives in patients with weak airway hyperresponsiveness8,9. Coffee, tea, cola, and chocolate taken on the test day can also affect the test results. Perpina et al10 reported that the cutoff value of 25 mg/mL for PC20 had a sensitivity of 87% and raising it to 70 mg/mL gave 100% sensitivity. Based on their study, the conventional methacholine challenge test could miss many asthma patients.
Asthma is a small airway disease and FEV1 is commonly used as a marker for bronchial narrowing in asthmatics. Although FEF25-75% also reflects small airway obstruction, it is not commonly used in real practice because it is less reproducible than FEV1. However, in healthy soldiers, FEF25-75% could be an early marker for small airway disease when FEV1 or FEV1/FVC were normal11, and FEF25-75% can be more sensitive in the methacholine bronchial provocation test12. One study in children with asthma showed that FEF25-75% was the most sensitive marker for detecting reduction in lung functions including FEV1 and FEV1/FVC13. In the methacholine provocation test in children, a 25% decrease of FEF25-75% revealed a close correlation with a 20% decrease of FEV114.
In this study, only one third of the patients were classified as having airway hyperresponsiveness by FEV1-PC20 criteria despite the history of current or recent wheezing. With such low positive rate of FEV1-PC20 in potential asthma patients, we needed more sensitive tool to detect mild airway hyperresponsiveness. Using a 20% fall in FEV25-75%, 20% more patients could be diagnosed with asthma. These patients were younger and had better basal lung function than the typical asthma group defined by FEV1-PC20 criteria. In addition, the average time on treatment was significantly shorter than typical asthma patients. Thus, patients with a 20% fall of FEF25-75% had a milder, earlier form of asthma.
Sputum eosinophilia reflects eosinophilic inflammation in the central airway and is frequently observed in asthma patients15-17. In this study, the percentage of patients with sputum eosinophilia showed a linear trend in the order of group I, II, and III. Although both group I and group II were classified as methacholine-negative by FEV1-PC20 criteria, group II had higher proportion of sputum eosinophil and reduction of lung function at 25 mg/mL of methacholine. Patients with sputum eosinophilia in group I and II could be diagnosed with eosinophilic bronchitis or atopic cough, but would have different clinical courses. In the literature, approximately 10% of eosinophilic bronchitis evolves to asthma18,19. We speculate that some patients in group II have an early, milder form of asthma and would eventually progress to typical asthma.
Figures and Tables
 | Figure 1Inter-group comparison of airway hyperresponsiveness. (A) Percent falls of FEV1 and FEF25-75% at 25 mg/mL of methacholine were significantly larger in group II than in group I. (B) †FEF25-75%-PC20 was about thrice higher in group II compared with group III. The results are expressed as mean±SEM. *p<0.001, t-test. †FEF25-75%-PC20 denotes provocative concentration of methacholine causing a 20% fall in FEF25-75%. |
 | Figure 2Association of airway hyperresponsiveness and sputum eosinophilia. (A) The patients with sputum eosinophilia more frequently had positive result in methacholine provocation test based on either FEV1 or FEF25-75% *p=0.002, †p=0.007, Pearson's chi-square. (B) The difference of FEV1-PC20 was insignificant between the patients with sputum eosinophilia and without sputum eosinophilia (p=0.203), but FEF25-75%-PC20 was significantly lower in the patients with sputum eosinophilia than in the patients without sputum eosinophilia (*p=0.032, t-test). The results are expressed as mean±SEM. |
References
1. Sistek D, Tschopp JM, Schindler C, Brutsche M, Ackermann-Liebrich U, Perruchoud AP, et al. Clinical diagnosis of current asthma: predictive value of respiratory symptoms in the SAPALDIA study. Swiss Study on Air Pollution and Lung Diseases in Adults. Eur Respir J. 2001. 17:214–219.
2. Cockcroft DW. Adkinson NF, Middleton E, editors. Chapter 73. Bronchial challenge testing. Middleton's allergy: principles & practice. 2009. 7th ed. Philadelphia: Mosby Elsevier;1295–1305.
3. Cockcroft DW, Murdock KY, Berscheid BA, Gore BP. Sensitivity and specificity of histamine PC20 determination in a random selection of young college students. J Allergy Clin Immunol. 1992. 89:23–30.
4. Song HJ, Chung JW, Choi JH, Suh CH, Nahm DH, Park HS. Clinical significance of bronchial hyperresponsiveness to adenosine 5-monophosphate in bronchial asthma. J Asthma Allergy Clin Immunol. 2004. 24:299–304.
5. Lee BJ, Min TH, Choi DC. Ever wheeze as a predictor of cough variant asthma. J Asthma Allergy Clin Immunol. 2004. 24:94–102.
6. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.
7. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000. 161:309–329.
8. Cockcroft DW, Davis BE, Todd DC, Smycniuk AJ. Methacholine challenge: comparison of two methods. Chest. 2005. 127:839–844.
9. Allen ND, Davis BE, Hurst TS, Cockcroft DW. Difference between dosimeter and tidal breathing methacholine challenge: contributions of dose and deep inspiration bronchoprotection. Chest. 2005. 128:4018–4023.
10. Perpina M, Pellicer C, de Diego A, Compte L, Macian V. Diagnostic value of the bronchial provocation test with methacholine in asthma: a Bayesian analysis approach. Chest. 1993. 104:149–154.
11. Marseglia GL, Cirillo I, Vizzaccaro A, Klersy C, Tosca MA, La Rosa M, et al. Role of forced expiratory flow at 25-75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007. 28:74–78.
12. Simon MR, Havstad S, Cotronei C, Krell W, Johnson CC, Peterson EL. Assessment of mid flow rate measurements in patients undergoing methacholine challenge. Allergy Asthma Proc. 2006. 27:404–410.
13. Lebecque P, Kiakulanda P, Coates AL. Spirometry in the asthmatic child: is FEF25-75 a more sensitive test than FEV1/FVC? Pediatr Pulmonol. 1993. 16:19–22.
14. Rhee KH, Kim JK, Kim JH, Lim DH, Son BK. Usefulness of FEF25-75% in methacholine bronchial provocation test in children with asthma. Pediatr Allergy Respir Dis. 2005. 15:408–414.
15. Keatings VM, Evans DJ, O'Connor BJ, Barnes PJ. Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluid. Thorax. 1997. 52:372–374.
16. Sosa IP, Nanulescu M. Induced sputum--means for detecting bronchial inflammation in children with atopic bronchial asthma and treatment monitoring. Pneumologia. 2004. 53:79–84.
17. Louis R, Bettiol J, Cataldo D, Bureau F, Seumois G, Radermecker M, et al. Value of induced sputum in the investigation of asthma. Rev Mal Respir. 2003. 20:215–223.
18. Berry MA, Hargadon B, McKenna S, Shaw D, Green RH, Brightling CE, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005. 35:598–601.
19. Park SW, Lee YM, Jang AS, Lee JH, Hwangbo Y, Kim DJ, et al. Development of chronic airway obstruction in patients with eosinophilic bronchitis: a prospective follow-up study. Chest. 2004. 125:1998–2004.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download