Abstract
Background
Elevated expression of cyclooxygenase-2 (COX-2) and Polo-like kinase-1 (PLK-1) is observed in a wide variety of cancers. Augmented expression of COX-2 and enhanced production of prostaglandin E2 (PGE2) are associated with increased tumor cell survival and malignancy; COX-2 has been implicated in the control of human non-small cell lung carcinoma (NSCLC) cell growth. PLK-1 siRNA induced the cell death of lung cancer cells and the systemic administration of PLK-1 siRNA/atelocollagen complex inhibited the growth of lung cancer in a liver metastatic murine model. COX-2 and PLK-1 are involved in proliferation and in cell cycle regulation, and there is a significant correlation between their interaction in prostate carcinoma.
Methods
In this study, we investigated the pattern of COX-2 and PLK-1 expression in NSCLC, after treatment with IL-1β, COX-2 inhibitor and PLK-1 siRNA.
Results
Expression of PLK-1 was decreased in A549 COX-2 sense cells, and was increased in A549 COX-2 anti-sense cells. Knock out of PLK-1 expression by PLK-1 siRNA augmented COX-2 expression in A549 and NCl-H157 cells. When A549 and NCI-H157 cells were treated with COX-2 inhibitor on a dose-dependent basis, PLK-1 and COX-2 were reduced. However, when the expression of COX-2 was induced by IL-1β, the production of PLK-1 decreased.
Figures and Tables
Figure 1
Expression of COX-2 and PLK-1 according to concentration of Interleukin (IL)-1β treatment. A549 (A) and NCI-H157 (B) cells were treated with 0, 0.015, 0.05, 0.1, 1, 1.5 ng/mL for 24 hours. Analysis of COX-2 and PLK-1 expression was done by Western blot. COX-2 protein expression was increased in dose dependent manner, but the expression of PLK-1 was decreased.

Figure 2
Expression of COX-2 and PLK-1 according to concentration of Interleukin (IL)-1β treatment. A549 and NCI-H157 cells were treated with 0.1, 1 ng/mL respectively for 0, 1, 2, 4, 8, 16, 20, 24 hours. Analysis of COX-2 and PLK-1 expression was done by Western blot. (A) In A549 cells, the maximum level of COX-2 expression was detected at 4 hour of exposure to IL-1β, and the minimum level of PLK-1 expression was detected from 4 hour to 8 hour of exposure to IL-1β. (B) In NCI-H157 cells, the maximum level of COX-2 expression was detected at 24 hour of exposure to IL-1β, and the minimum level of PLK-1 expression was detected at 16 hour of exposure to IL-1β.

Figure 3
Expression of COX-2 and PLK-1 according to concentration of celecoxib treatment. A549 (A) and NCI-H157 (B) cells were treated with 0, 10, 20, 30, 40, 50 µM (Land 1~6) for 24 hours. Analysis of COX-2 and PLK-1 expression was done by western blot. Expression of PLK-1, as well as COX-2, was decreased in dose dependent manner.

Figure 4
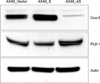
Expression of COX-2 and PLK-1 in COX-2 gene modified human adenocarcinoma cell lines. According to the expression of COX-2 is increase, PLK-1 expression is decreased. A549-vector: A549 COX-2 vector only cell line; A549-S: A549 COX-2 sense cell line; A549-AS: A549 COX-2 anti-sense cell line.
References
1. Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol. 2000. 165:2719–2728.
2. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993. 90:11693–11697.
3. Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990. 265:10805–10808.
4. Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992. 267:25934–25938.
5. Hasturk S, Kemp B, Kalapurakal SK, Kurie JM, Hong WK, Lee JS. Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung carcinoma. Cancer. 2002. 94:1023–1031.
6. Yoon JM, Lim JJ, Yoo CG, Lee CT, Han SK, Shim YS, et al. The role of uteroglobin in the immunomodulation of nonsmall cell lung cancer cells. Tuberc Respir Dis. 2004. 57:336–344.
7. Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006. 98:736–747.
8. Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006. 12:5113–5121.
9. Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993. 90:4882–4886.
10. Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988. 89:25–38.
11. Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998. 10:776–783.
12. Donaldson MM, Tavares AA, Hagan IM, Nigg EA, Glover DM. The mitotic roles of Polo-like kinase. J Cell Sci. 2001. 114:2357–2358.
13. Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997. 14:543–549.
14. Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999. 15:687–692.
15. Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002. 29:354–358.
16. Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000. 196:753–759.
17. Dietzmann K, Kirches E, von B, Jachau K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001. 53:1–11.
18. Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett. 2001. 164:41–49.
19. Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett. 2001. 169:41–49.
20. Kawata E, Ashihara E, Kimura S, Takenaka K, Sato K, Tanaka R, et al. Administration of PLK-1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer. Mol Cancer Ther. 2008. 7:2904–2912.
21. Denkert C, Thoma A, Niesporek S, Weichert W, Koch I, Noske A, et al. Overexpression of cyclooxygenase-2 in human prostate carcinoma and prostatic intraepithelial neoplasia-association with increased expression of Polo-like kinase-1. Prostate. 2007. 67:361–369.
22. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000. 30:3–21.
23. Jang JW. Anti-tumor mechanisms and regulation of survivin by selective cyclooxygenase-2 inhibitor. Korean J Hepatol. 2008. 14:305–308.
24. Peri A, Cordella-Miele E, Miele L, Mukherjee AB. Tissue-specific expression of the gene coding for human Clara cell 10-kD protein, a phospholipase A2-inhibitory protein. J Clin Invest. 1993. 92:2099–2109.
25. Yoon JH, Kim KS, Kim HU, Linton JA, Lee JG. Effects of TNF-alpha and IL-1 beta on mucin, lysozyme, IL-6 and IL-8 in passage-2 normal human nasal epithelial cells. Acta Otolaryngol. 1999. 119:905–910.
26. Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference. Airway inflammation. Ann Intern Med. 1995. 123:288–304.
27. Lin CH, Sheu SY, Lee HM, Ho YS, Lee WS, Ko WC, et al. Involvement of protein kinase C-gamma in IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol Pharmacol. 2000. 57:36–43.
28. Morton RS, Dongari-Bagtzoglou AI. Cyclooxygenase-2 is upregulated in inflamed gingival tissues. J Periodontol. 2001. 72:461–469.
29. Laporte JD, Moore PE, Panettieri RA, Moeller W, Heyder J, Shore SA. Prostanoids mediate IL-1beta-induced beta-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am J Physiol. 1998. 275:L491–L501.
30. Pang L, Knox AJ. Effect of interleukin-1 beta, tumour necrosis factor-alpha and interferon-gamma on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol. 1997. 121:579–587.
31. Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000. 141:4664–4670.
32. Kim YD, Song SY, Kwon EJ, Baek SH, Cho GS, Kim HS, et al. IL-1beta mediated COX-2 expression in human airway epithelial cells. Korean J Otolaryngol - Head Neck Surg. 2002. 45:132–136.
33. Pyrko P, Soriano N, Kardosh A, Liu YT, Uddin J, Petasis NA, et al. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer. 2006. 5:19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download