Abstract
Delftia acidovorans is a gram-negative motile rod found ubiquitously in soil and in water. Confirmed isolation from clinical infections is rare, and has been documented mostly in immunocompromised patients or those with indwelling catheters. A 53-year-old man was referred for the evaluation of a huge mass-like lesion found incidentally by chest X-ray. The lesion occupied more than half of the right lung and was diagnosed as a large loculated pleural effusion by CT scan. Bloody pus was drained through a percutaneous catheter, and D. acidovorans, identified by the Vitek GN card and confirmed by amplification of 16S ribosomal RNA and sequencing analysis, was isolated repeatedly from the drained pus. The patient was treated with imipenem/cilastatin to which the organism was sensitive. This is a rare report of chronic empyema associated with D. acidovorans in the respiratory system of an immunocompetent patient.
Delftia acidovorans, formerly called as Comamonas acidovorans, is a gram-negative aerobic rod found ubiquitously in soil and water1, and has seldom been implicated in human infections. It has occasionally been reported in cases of catheter-related bacteremia2,3, peritonitis in patients on continuous ambulatory peritoneal dialysis4, ocular infection5, endocarditis6, and infections in patients with AIDS7. It was also identified from some isolates referred to as "Burkholderia cepacia" from cystic fibrosis clinics and research8.
We report a rare isolation of D. acidovorans from an immunocompetent patient with chronic empyema.
A 53-year-old man was referred for evaluation of a mass-like lesion in his right lung, detected by a chest X-ray. He was a 50 pack-year, current smoker and had no symptoms except for mild intermittent chest discomfort. He had been injured while working in the construction industry about 25 years earlier and was tapped repeatedly at the incident site for a pleural effusion. Thereafter, he felt healthy and reported no medical problems.
On admission, the patient's body temperature was 36.7℃ and chest expansion on breathing was asymmetric with decreased breath sounds on the right lung. In the initial laboratory tests, the leukocyte count (4,900 cells/mm3), erythrocyte sedimentation rate (9 mm/h), and C-reactive protein (0.08 mg/dL) were within normal limits. On chest X-ray, there was a huge mass-like lesion in the right lower lung field (Figure 1A). On a CT scan of the chest, there was a 15×19×26 cm sized, multiloculated lesion with low-attenuated components and thickening of the calcified wall, indicating chronic empyema (Figure 1B).
Chocolate-like bloody pus was drained from the lesion by diagnostic thoracentesis. It showed a protein of 10.2 g/dL, LDH of 3,287 U/L, hemoglobin of 2.7 g/dL and the leukocyte count of 188,500 cells/mm3. Gram stain and culture using agar-based culture media were negative. AFB stain and culture, and tuberculosis PCR of the pus were also negative, and the pus was drained by two percutaneous catheters. The catheter in the anteromedial septated cavity was removed on the eighth day of hospitalization but the other in the inferolateral side was left in place because 100~200 mL of pus daily continued to drain.
On the fifteenth day, the patient presented fever. The leukocyte count and C-reactive protein were elevated to 11,500 cells/mm3 and 3.12 mg/dL, respectively. After cultures of both pus drained through the catheters and blood using blood culture bottles (BACTEC™, Becton Dickinson [BD], Sparks, MD, USA), intravenous moxifloxacin was commenced for suspected hospital-acquired pneumonia, but clinical findings worsened with persistent fever for three days. The repeated CT scan showed that the empyematous cavity in the anteromedial side remained (Figure 1C). Another catheter was inserted into the enlarged loculated empyema, and the antibiotics was changed to imipenem/cilastin 500 mg every 6 h intravenously.
D. acidovorans was isolated from the pus after the incubation period of five days, while blood culture yielded no growth. The organism was identified by the Vitek GN card (BioMérieux Vitek Inc., Hazelwood, MO, USA). The antibiotic susceptibility was performed by the Vitek AST N055 card, an automated susceptibility testing system, and determined by Clinical and Laboratory Standards Institute (CLSI) guidelines. The antibiogram showed that the organism was susceptible to cefotaxime, ceftazidime, cefepime, piperacillin-tazobactam, trimethoprim-sulfamethoxazole and imipenem, but resistant to amikacin, gentamycin and tobramycin, with intermediate resistance to ciprofloxacin.
As a reference testing method, the presence of D. acidovorans was confirmed by amplification and sequencing of 16S ribosomal RNA (rRNA). PCR was performed using universal primers (27F: 5'-AGAGTTTGATCCTGGCTCAG-3', 1492R: 5'-AAGGAGGTGATCCAGCCGCA-3') designed to anneal a conserved position in the 30 and 50 regions of bacterial 16S rRNA genes9. The PCR products were electrophoresed and purified with a QIAEX II gel extraction kit (QIAGEN, Hilden, Germany) (Figure 2), and sequenced with an Applied Biosystems automated sequencer (model 377) and BigDye Terminator cycle sequencing kits (Perkin-Elimer Applied Biosystems, Warrington, UK). The gene sequences were analyzed by Basic Local Alignment Search Tool (Figure 3).
D. acidovorans was repeatedly isolated from the drainage via the catheters by three additional tests using the blood culture bottles after the first isolation. No other organisms were identified from the drained pus and blood samples. Clinical symptoms were improved after administration of appropriate antibiotics and effective drainage. The percutaneous catheters were removed after the loculated empyema had almost resolved, at which time imipenem/cilastin had been administered for four weeks. The patient was discharged on the 43rd day of hospitalization.
D. acidovorans is still considered as an unusual pathogen in human respiratory system. This is the rare report of its isolation from the pleural cavity in active infection.
It is important to consider whether the isolated bacterium is a true pathogen of the active infection or a contaminant of the indwelling catheters. The observations that the microorganism was isolated from the drainage when the patient was suffering from active infection, and it was the sole organism repeatedly isolated led us to believe that it was a pathogen.
D. acidovorans was not isolated from the initial drainage. It suggests that the organism was the etiologic agent of secondary infection related with a percutaneous catheter rather than that of chronic empyema. However, the bloody exudate drained by initial thoracentesis was cultured using agar-based culture media. Thereafter, the organism was repeatedly isolated by blood culture bottles. The method using the blood culture bottles produced a significant and consistent increase in the isolation rate for body fluid cultures compared to conventional method10. Therefore, we cannot exclude that D. acidovorans may be the causative agent for the empyema. It has been reported that D. acidovorans infection is associated with exposure to contaminated venous catheters11. We presume that when he had a chest injury and been tapped repeatedly, the organism might be inoculated. In this case, the chronic asymptomatic empyema could progress slowly because D. acidovorans has a low pathogenicity to immunocompetent patients. However, the evidence about the true pathogen of empyema is limited due to no isolation of initial culture.
Because D. acidovorans is often resistant to a class of drugs commonly used to treat gram-negative infections such as aminoglycosides, timely identification of this organism is necessary to determine the appropriate antibiotics11. In this case, imipenem/cilastatin was maintained for four weeks as a routine duration of treatment for chronic empyema, and the patient recovered from active infection after changing to susceptible antibiotics combined with effective drainage.
This is a rare report on an immunocompetent patient with chronic empyema associated with D. acidovorans treated with appropriate antibiotics and therapeutic drainage.
Figures and Tables
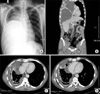 | Figure 1Radiologic examinations. (A) At admission, a huge round lesion was observed in the right lower lung field at PA view of chest X-ray. (B) The lesion was identified by chest CT scan as a huge septated empyema with thickening of the calcified wall and passive atelectasis of the right middle and lower lobes. (C) On the 18th day of hospitalization, a CT scan showed that the empyematous cavity in the anteromedial side of the right lower lung field remained. (D) A chest CT scan was repeated three weeks later, showing that the size of the cavity was decreased after insertion of percutaneous catheter in the cavity, guided by fluoroscope. |
References
1. Bergogne-Bérézin E. Cohen J, Powderly W, editors. Pseudomonas and miscellaneous gram-negative bacilli. Infectious disease. 2004. 2nd ed. St Louis: Mosby;2203–2217.
2. Castagnola E, Conte M, Venzano P, Garaventa A, Viscoli C, Barretta MA, et al. Broviac catheter-related bacteraemias due to unusual pathogens in children with cancer: case reports with literature review. J Infect. 1997. 34:215–218.
3. Ender PT, Dooley DP, Moore RH. Vascular catheter-related Comamonas acidovorans bacteremia managed with preservation of the catheter. Pediatr Infect Dis J. 1996. 15:918–920.
4. López-Menchero R, Sigüenza F, Caridad A, Alonso JC, Ferreruela RM. Peritonitis due to Comamonas acidovorans in a CAPD patient. Perit Dial Int. 1998. 18:445–446.
5. Stonecipher KG, Jensen HG, Kastl PR, Faulkner A, Rowsey JJ. Ocular infections associated with Comamonas acidovorans. Am J Ophthalmol. 1991. 112:46–49.
6. Horowitz H, Gilroy S, Feinstein S, Gilardi G. Endocarditis associated with Comamonas acidovorans. J Clin Microbiol. 1990. 28:143–145.
7. Franzetti F, Cernuschi M, Esposito R, Moroni M. Pseudomonas infections in patients with AIDS and AIDS-related complex. J Intern Med. 1992. 231:437–443.
8. Henry D, Campbell M, LiPuma J, Speert D. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997. 35:614–619.
9. Massol-Deya A, Odelson D, Hickey R, Tiedje J. Akkermans ADL, Van Elsas JD, De Bruijn FJ, editors. Bacterial community fingerprinting of amplified 16S and 16-23S ribosomal DNA gene sequences and restriction endonuclease analysis. Molecular microbial ecology manual. 1995. Boston: Kluwer Academic;1–8.
10. Daur AV, Klimak F Jr, Cogo LL, Botao GD, Monteiro CL, Dalla Costa LM. Enrichment methodology to increase the positivity of cultures from body fluids. Braz J Infect Dis. 2006. 10:372–373.
11. Perla R, Knutson E. Delftia acidovorans bacteremia in an intravenous drug abuser. Am J Infect Dis. 2005. 1:73–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download