Isoniazid is an essential drug in antituberculous regimens against Mycobacterium tuberculosis. The serious adverse effects of isoniazid are not common and include hepatitis, peripheral neuropathy, and cutaneous reactions1. Although isoniazid has also been involved as a cause of gynecomastia2, the reported cases are fairly rare3-6. We describe here a case of gynecomastia that had isoniazid as causative agent.
A 72-year old, man was admitted to the hospital with a complaint of one month of productive cough. The patient had been left paraplegic since 1988, when fell down by accident. He denied tobacco, alcohol, or any drug use. Given her history, primary care physician treated supportively with antibiotics for presumed pneumonia. This provided no symptomatic relief. He experienced no fever, weight loss, night sweats, chest pain or hemoptysis.
Physical examination revealed a thin-appearing old man with no apparent respiratory distress. He was awake and oriented. On auscultation of lung, breath sound was decreased over right upper lung. Cardiac and skin examinations were normal, as was the remainder of the physical examination.
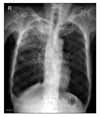
Laboratory data revealed the following: WBC count, 11,100/mm3 with 82% neutrophil and 8% lymphocyte; hemoglobin, 12.8 g/dl; hematocrit, 37.0%; C-reactive protein, 2.49 mg/dl; albumin, 3.3 g/dl; aspartate aminotransferase, 47 IU/L; alanine aminotransferase, 56 IU/L; alkaline phosphatase, 131 IU/L; total bilirubin, 0.3 mg/dl; sodium, 134 mEq/L; potassium, 4.3 mEq/L; chloride, 107 mEq/L; urea nitrogen, 39 mg/dl; creatinine 2.1 mg/dl; and creatinine clearance 20.6 ml/min. Thyroid function test was normal. A urinalysis showed negative protein, 5 to 9 RBCs, and 1 to 4 WBCs per high-power field. The patient was diagnosed with stable chronic kidney disease at nephrology consultation. Regular follow up was planned without dialysis. Arterial blood gas analysis showed a pH of 7.340; PaCO2, 27.0 mm Hg; PaO2, 95.0 mm Hg; and oxygen saturation, 97.0% while he was breathing ambient air. Laboratory evaluation for Legionella, pneumococcal and Mycoplasma were negative. Tuberculin skin test and HIV serology were also negative. Chest X-ray (Figure 1) revealed a patchy consolidation with air bronchogram on right upper lung field.
Treatment was initiated with antibiotics for a presumed pneumonia. Sputum smear for acid fast bacilli and polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative. Bronchoscopy showed anthracotic pigmentation in the whole bronchus with some secretions in right upper lobe. Only PCR for Mycobacterium tuberculosis in bronchial washing was positive.
The patient was started on antituberculous therapy, with isoniazid (300 mg daily), rifampin (600 mg daily), ethambutol (400 mg thrice weekly), and pyrazinamide (1,000 mg thrice weekly) in appropriate doses in view of his creatinine clearance results. After taking the medicine, he had improved with gradual resolution of constitutional symptoms without any adverse reaction of antituberculous medicine. He was discharged on admission day 23. Subsequent culture of sputum, bronchial washings and urine were negative for Mycobacterium tuberculosis.
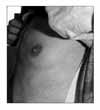
He was seen in outpatient clinic at which time he reported that he was doing well. Repeated chest radiography revealed also slightly improved infiltration on right upper lung. After four months, while receiving only EHR, he noticed a swelling around the both nipples (Figure 2) with an otherwise normal physical examination. Approximately 4 cm in diameter, the lumps felt rubbery and slightly tender. Mammography showed proliferation of fibronodular tissue in both subareolar areas with prominent gynecomastia on left side. Ultrasonogram of breast revealed no definite lesion. He reported the recent onset of breast enlargement during antituberculous therapy and receiving one of possible causative drugs causing gynecomastia. Isoniazid was stopped immediately with a presumptive diagnosis of isoniazid associated gynecomastia. Antituberculous regimen except for isoniazid was given without further diagnostic procedure. His breast swelling and tenderness have resolved slowly within 1 month. Patient has completed a 9-months course of antituberculous treatment and is now in follow-up. His gynecomastia has disappeared completely 1.5 years after cessation of isoniazid.
Gynecomastia is a disorder resulting from hypertrophy of male breast, that has been reported to occur in up to 40% to 65% of men7,8. In adult gynecomastia, 25 percent had persistent pubertal gynecomastia, 10 to 25 percent had drug-induced gynecomastia, and an additional 25 percent were considered to have idiopathic gynecomastia2.
Once the diagnosis of gynecomastia is established, it is important to review all medications. Many drugs can cause gynecomastia, including drugs that decrease testosterone synthesis such as ketoconazole, metronidazole, or cytotoxic agents and drugs that decrease testosterone action such as marijuana, cimetidine, and spironolactone. Some drugs such as verapamil, nifedipine, amiodarone, tricyclic antidepressants, furosemide, theophylline, isoniazid can cause gynecomastia via an unknown mechanism of action9.
Gynecomastia may be also seen in systemic conditions such as obesity, liver disease, renal disease and hyperthyroidism. Although our patient had also chronic kidney disease, we attributed the gynecomastia to isoniazid, as developed during antituberculous therapy including isoniazid and improved after cessation of that drug. The patient's renal function also remained stable without requiring dialysis during the entire antituberculous treatment.
When the patient's history and physical examination do not reveal the cause of gynecomastia, endocrine screening test should be measured7. It has been documented that routine endocrine evaluation of all patients with idiopathic gynecomastia is not necessary2. We didn't undertake endocrine evaluation since the secondary sexual characters and the external genitalia were found to be normal in our case. He also reported the recent onset of breast enlargement during antituberculous therapy and receiving one of possible causative drugs causing gynecomastia. In addition, there was definitely a decrease in breast enlargement after discontinuing isoniazid.
In 1953, a first report from France involved isoniazid as a cause of gynecomastia3. Another French report described painless, bilateral gynecomastia in a man who was receiving 600 mg of isoniazid daily for four months4. In 2003, a report described bilateral painful gynecomastia after 4 months of isoniazid (300 mg daily)5. A recent report described painful swelling in left breast after four months of isoniazid (600 mg thrice weekly)6. Our patient developed bilateral painful gynecomastia after four months therapy with isoniazid (300 mg daily). In most reported cases, onset of breast enlargement occured four months after antituberculous therapy, although prescribed doses were different.
Currently there is no evidence to account for a mechanism for the development of gynecomastia with isoniazid. Among other antituberculosis drugs, thiacetazone and ethionamide have been reported in a case report as a cause of gynecomastia1,10. Although our patient was also on rifampin and ethambutol, there have been no reports involving these drugs.
Although treatment of idiopathic gynecomastia is difficult, the treatment of gynecomastia is predicated on the underlying cause. If gynecomastia is related to a particular drug exposure as in our case, the offending drug should be discontinued.
Figures and Tables
References
1. Girling DJ. Adverse effects of antituberculosis drugs. Drugs. 1982. 23:56–74.
2. Braunstein GD. Gynecomastia. N Engl J Med. 1993. 328:490–495.
3. Guinet P, Garin JP, Morneix A. Gynecomastia in a grave case of pulmonary tuberculosis during isonicotinic acid hydrazide therapy. Lyon Med. 1953. 188:281–284.
4. Bergogne-Berezin E, Nouhouayi A, Letonturier P, Thibault B, Tourneur R. Gynecomastia caused by isoniazid. Value of determination of the inactivation phenotype. Nouv Presse Med. 1976. 5:213–214.
5. Khanna P, Panjabi C, Maurya V, Shah A. Isoniazid associated painful, bilateral gynecomastia. Indian J Chest Dis Allied Sci. 2003. 45:277–279.
6. Dixit R, Sharma S, Nawal CL. Gynaecomastia during antituberculosis chemotherapy with isoniazid. J Assoc Physicians India. 2008. 56:390–391.
7. Williams MJ. Gynecomastia: its incidence, recognition and host characterization in 447 autopsy cases. Am J Med. 1963. 34:103–112.
8. Bowers SP, Pearlman NW, McIntyre RC Jr, Finlayson CA, Huerd S. Cost-effective management of gynecomastia. Am J Surg. 1998. 176:638–641.
9. Thompson DF, Carter JR. Drug-induced gynecomastia. Pharmacotherapy. 1993. 13:37–45.
10. Chunhaswasdikul B. Gynecomastia in association with administration of thiacetazone in the treatment of tuberculosis. J Med Assoc Thai. 1974. 57:323–327.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download