Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis and the transmission occurs nearly always through an airborne route1. The length of time exposed to air contaminated with M. tuberculosis, and the extent of intimate exposure are regarded as important factors increasing its risk of transmission of M. tuberculosis1. Close contacts living environments with active pulmonary tuberculosis patient increase the time exposed to M. tuberculosis. Patients with active pulmonary tuberculosis produce and expel droplet nuclei holding tubercle bacilli into the air which remain suspended unless ventilation is performed effectively. This can greatly increase the risk of M. tuberculosis transmission during close contact1. Conventional contact investigation has been used to identify contact persons with high transmission risks2. Genotyping of M. tuberculosis is a very effective way to evaluate contact investigations for transmission3. After the introduction of M. tuberculosis genotyping, epidemiologic studies reported that conventional contact investigations compared to genotyping underestimated transmission rates because casual transmission was hard to detect with traditional contact tracing4,5. On the contrary, a case series showed that conventional contact investigations compared to the genotyping approach actually overestimated transmission rates because 5 of 14 isolates proved not to be acquired from the expected source6. We report a case of intrafamilial transmission of M. tuberculosis was clearly identified by both conventional contact investigation and genotyping.
A 57-year-old male presented to our emergency department complaining of dyspnea (NYHA Classification IV). He was a 40 pack-year ex-smoker with a past medical history significant for diabetes and myocardial infarction. The patient had been prescribed HERZ anti-tuberculous medication (Isoniazid 300 mg, Rifampicin 450 mg, Ethambutol 600 mg, and Pyrazinamide 1,000 mg once daily) for the past month after being diagnosed with pulmonary tuberculosis at a primary care clinic. On examination, he was found to have a left sided pleural empyema. Acid-fast bacilli (AFB) were found in the pleural effusion as well as sputum. Subsequent AFB cultures were positive for M. tuberculosis and sensitivity testing revealed sensitivity to all anti-tuberculosis medications. Contrastenhanced CT of the chest at the time of diagnosis of pulmonary tuberculosis revealed multiple, variable sized cavity lesions and multiple small nodules with branching opacities in both upper lobes.
To investigate the potential transmission of tuberculosis within his family members, the spouse and three sons of the patient were asked to visit our hospital for the detailed examinations. His spouse agreed, but the three sons declined because they recently all had unremarkable health examinations, to include a chest X-ray without sputum analysis. The wife reported no prior tuberculosis infections or pulmonary symptoms. The mean time of exposure to her husband, the "source patient", was calculated to be 9 hours daily (mealtimes, watching TV, sleeping). The spouse of "contact patient" on physical exam appeared relatively healthy except for a low body mass index of 1.64. After further evaluation, nodular opacities were shown in the right upper lobe by chest radiography (Figure 1). Chest CT demonstrated active pulmonary tuberculosis characterized by small cavities and small nodules with branching opacities. Her sputum AFB smear was negative, but eventually grew positive AFB cultures. Subsequent analysis of the AFB revealed sensitivities for all anti-tuberculosis drugs. She was also administered with HREZ therapy, which included Isoniazid 300 mg, Rifampicin 450 mg, Ethambutol 600 mg, and Pyrazinamide 1,000 mg once daily.
IS6110 Restriction Fragment Length Polymorphism (RFLP) for the isolates was performed separately for the two cases at the Korean National Tuberculosis Association to ascertain whether the husband's M. tuberculosis was the transmitted source. The pattern of RFLP proved to be same between the two (Figure 2).
In according to the WHO 2002 Tuberculosis Surveillance, the incidence of tuberculosis in Korea was estimated as 91 per 100,000 people. The incidence of Korea was higher than most advanced countries, which were estimated at 5 per 100,000 people in US and 14 per 100,000 people in France7. In addition, new cases of tuberculosis in Korea were reported at 65.4 per 100,000 people in 2004, with the peak incidence of new cases occurring in the twenties and over 65 year-old age populations8. The high incidence of tuberculosis in young Korean implies that tuberculosis infection transmission risk remains high8.
M. tuberculosis is transmitted through the airborne route, and once effective multipledrug chemotherapy is initiated, the infectivity of patients rapidly decreases9,10. From the standpoint of infection transmission, there is substantial evidence that positive sputum smears and close contact can synergistically increase the rate of tuberculosis infection2,11,12. In a previous contact investigation study of 1,590 established contacts, the prevalence of tuberculosis was significantly higher with 46.4% in persons who were contacted with household shown positive sputum smears compared with those who were not contact with household shown positive sputum smears, those who were contacted with household with negative one, and those who were contacted with non-household shown with negative one, 34%, 28%, and 24.5%, respectively2. In another study with 2,941 contacts with tuberculosis cases were analyzed. Regarding the relationship between the contacts and infection transmission, positive AFB smear, cavitary disease, and total hours exposed to the infected individual each month were closely related to conversion to positive tuberculin skin test (TST)12. In addition, the number of optimal sized infectious particles suspended in the air, the duration of exposure, and the virulence of the organism are associated with the acquisition of tuberculosis infection13. About 10% of persons who acquired tuberculosis infection may develop active tuberculosis unless there is adequate chemical prophylaxis. The risk for progression to disease is the highest within the first 2 years after infection, when approximately half of the cases will occur14.
In our case, the source patient (husband) was considered to have highly infectious tuberculosis given the positive sputum smear and multiple cavities in both of his lung fields. The close contact patient was exposed to as long as 9 hours a day to the source patient. The high burden of M. tuberculosis of the source patient and long-standing exposure to him may have rendered the close contact patient to be infected. It is uncertain whether our close contact patient's low BMI played a role in the development of active tuberculosis since the risk of tuberculosis was increased by 2.8 times in patients with a body mass index less than 20 in one study15.
Interferon-γ-release assays (IGRAs) currently available, such as T-SPOT TB (Oxford Immunotec, Oxford, United Kingdom) measuring peripheral blood mononuclear cells which produce IFN-γ, and QuantiFERON-TB Gold or in tube test (Cellestis, Victoria, Australia) measuring antigen-specific production of interferon-γ in circulating T cells of whole blood, showed excellent specificity (96~99%) and suboptimal sensitivity (70~90%), However, its use is confined to detect patients with latent tuberculosis rather than to clarify tuberculosis transmission16,17. Moreover, a study performed in South Africa known as a high incidence area (320 per 100,000) showed that only 81 of 433 contacts (19%) were considered as tuberculosis transmission within the household18. We therefore used molecular genotyping of M. tuberculosis isolates to clarify the disease transmission from the source patient to the close contact. To investigate transmission pattern between the source and contact case, the IS6110 restriction fragment length polymorphism (RFLP) analysis was used as the standardized molecular typing method for M. tuberculosis isolates19. Since studies for tuberculosis outbreak investigation proved that identical DNA fingerprints of M. tuberculosis isolates were shared with cases, identical ones recovered from different patients have been considered as recent transmission and unique patterns have been assumed to be from the reactivation of latent disease20. Since both of them showed the identical RFLP patterns and DNA fingerprints of M. tuberculosis isolates epidemiologically connected generally show identical RFLP patterns3, tuberculosis in the contact case may have acquired by recent transmission from the source case rather than from the reactivation of latent tuberculosis.
In a genotyping study with 138 Korean M. tuberculosis isolates, 2 cases were already reported to be transmitted through household contact and neighborhood contact21. Based on a history of close contact with patient with high infectivity, identical molecular genotyping of M. tuberculosis isolates, and the same anti-tuberculosis sensitivity profiles, our contact case is considered to be caused by recent intra-familial transmission of tuberculosis. In spite of more advanced approach to clarify transmission patterns of tuberculosis, the major limitation of our case study is that we confined our investigations for conventional contact tracing and DNA fingerprinting of M. tuberculosis isolates only to one family.
Figures and Tables
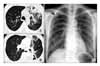
Figure 1
High Resolution CT (HRCT) of the chest in the source patient case revealed multiple, variable sized cavity lesions and multiple small nodules with branching opacities in both lungs at the time of diagnosis with active pulmonary tuberculosis. (A, B) There were well encapsulated pleural collections and diffuse pleural thickening in the left hemithorax. (C) Routine chest radiography in the close contact patient showed a small cavity with nodules in right upper lung field.
References
1. American Thoracic Society. Centers for Disease Control and Prevention. Infectious Diseases Society of America. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005. 172:1169–1227.
2. Rose CE Jr, Zerbe GO, Lantz SO, Bailey WC. Establishing priority during investigation of tuberculosis contacts. Am Rev Respir Dis. 1979. 119:603–609.
3. Barnes PF, Cave MD. Molecular epidemiology of tuberculosis. N Engl J Med. 2003. 349:1149–1156.
4. Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med. 1994. 330:1703–1709.
5. McNabb SJ, Kammerer JS, Hickey AC, Braden CR, Shang N, Rosenblum LS, et al. Added epidemiologic value to tuberculosis prevention and control of the investigation of clustered genotypes of Mycobacterium tuberculosis isolates. Am J Epidemiol. 2004. 160:589–597.
6. Dahle UR, Nordtvedt S, Winje BA, Mannsaaker T, Heldal E, Sandven P, et al. Tuberculosis in contacts need not indicate disease transmission. Thorax. 2005. 60:136–137.
7. World Health Organization. Global tuberculosis control: surveillance, planning, financing. WHO Report 2004. 2004. Geneva: World Health Organization.
8. Korea Center for Disease Control and Prevention, Korean Institute of Tuberculosis. Annual report on the notified tuberculosis patients in Korea. 2005. Seoul: Korea Center for Disease Control and Prevention, Korean Institute of Tuberculosis.
9. Kamat SR, Dawson JJ, Devadatta S, Fox W, Janardhanam B, Radhakrishna S, et al. A controlled study of the influence of segregation of tuberculous patients for one year on the attack rate of tuberculosis in a 5-year period in close family contacts in South India. Bull World Health Organ. 1966. 34:517–532.
10. Gunnels JJ, Bates JH, Swindoll H. Infectivity of sputum-positive tuberculous patients on chemotherapy. Am Rev Respir Dis. 1974. 109:323–330.
11. Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975. 50:90–106.
12. Bailey WC, Gerald LB, Kimerling ME, Redden D, Brook N, Bruce F, et al. Predictive model to identify positive tuberculosis skin test results during contact investigations. JAMA. 2002. 287:996–1002.
13. Leff A, Geppert EF. Public health and preventive aspects of pulmonary tuberculosis: infectiousness, epidemiology, risk factors, classification, and preventive therapy. Arch Intern Med. 1979. 139:1405–1410.
14. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000. 161:1376–1395.
15. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006. 55:19–26.
16. van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993. 31:406–409.
17. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008. 149:177–184.
18. Verver S, Warren RM, Munch Z, Richardson M, van der Spuy GD, Borgdorff MW, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004. 363:212–214.
19. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007. 146:340–354.
20. Geng E, Kreiswirth B, Driver C, Li J, Burzynski J, DellaLatta P, et al. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N Engl J Med. 2002. 346:1453–1458.
21. Park YK, Bai GH, Kim SJ. Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from countries in the western pacific region. J Clin Microbiol. 2000. 38:191–197.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download