Abstract
We treated synchronous double primary lung cancers, where one site resulted from CIS disease, with lobectomy and argon plasma coagulation (APC) in a patient who couldn't tolerate pneumonectomy, which resulted in a reduction of the extent of surgery. APC could be a reasonable alternative for CIS disease of lung in inoperable patients.
The development of the autofluorescence bronchoscopy (AFB) technique in the early 1990s in addition to traditional sputum cytology methods enabled early detection of both bronchial intraepithelial neoplasia and lung cancer in central airways. Even though this advancement allowed for earlier intervention, it unfortunately did not achieve significantly increased survival rates1. Recently the prevalence of synchronous roentgenographically occult lung carcinoma (ROLC) in patients with resectable primary lung cancer was determined to be relatively high, 9.3%, by AFB and biopsy2. Carcinoma in situ (CIS) diseases from the bronchus are known to progress to invasive carcinomas in more than half of the cases. Surgical resection remains the primary curative treatment of lung cancer. However, several endobronchial treatment modalities are available for curative or palliative purpose in inoperable patients with limited cardiopulmonary reserve3. Synchronous second primary lung cancer in non small cell lung cancer (NSCLC), is not a rare phenomenon and current guidelines recommend considering curative surgical resection for both of types of lesions, invasive mediastinal staging and extrathoracic imaging. We report a case of synchronous double primary lung cancers that were treated with lobectomy for lung mass and argon plasma coagulation (APC) for another lesion of CIS.
A 68 year-old male patient visited our hospital for evaluation of an incidental right upper lung mass that was found in a chest x-ray performed at a routine check-up. The patient was a current smoker with 15 pack-years of smoking history. In addition, he had been diagnosed with hypertension and diabetes mellitus and was on regular medications. He was free from any respiratory symptoms, and had no systemic complaints such as weight loss, general weakness and decreased appetite.Furthermore, the patient's physical examination revealed no abnormal findings and his vital sign was stable upon admission. Laboratory findings revealed a white cell count of 9.21×109/L, 17.9 g/dL hemoglobin, 50.7% hematocrit, and platelet count 227×109/L. Routine chemistry and ABGA were also unremarkable. However in the chest x-ray the right hilum was prominent, and the chest dynamic CT showed 3.7×2.7 cm sized heterogenously enhanced low density mass that completely obstructed the anterior segmental bronchus of the right upper lobe (Figure 1). We performed autofluorescence bronchoscopy (OncoLIFE®, Xillix, British Columbia) and obtained a tissue sample, using forcep biopsy, from the obstructing intraluminal mass in the right upper lobe (Figure 2A) and the superior segment of the right lower lobe where a loss of autofluorescence was observed without mucosal abnormalities (Figure 2B). The pathology reports indicated that the mass in the right upper lung was squamous cell carcinoma and the lesion in the superior segment consisted of squamous cell carcinoma in situ (Figure 3). No distant metastasis was observed in the brain MRI, whole body bone scan, and whole body PET/CT. In the preoperative physiologic evaluation, the patient's performance was good and the postbronchodilator FEV1 and MVV were 2.39 L (92.2% of pred), and 61.8 L (60.4% of pred), respectively, therefore the right pneumonectomy was planned. During the operation, the right lobectomy and mediastinal lymph node dissection were done because he could not tolerate one-lung ventilation. After 3 weeks, the superior segment of the right lower lung lesion was treated with one session of APC (ERBE®, Elektromedizin Tübingen, Germany) and no immediate complication was encountered (Figure 2C, D). On the 18th day post-APC, an ulcerative lesion with mild edema was observed by bronchoscopy (Figure 2E), and the biopsy report revealed acute and chronic inflammation. He was discharged uneventfully and there was no evidence of recurrence in a follow-up bronchoscopy at 3 month post-APC (Figure 2F), and in CT at 1 year post-APC.
Previously, bronchoscopic intervention was used for the removal of foreign bodies or toileting bronchial secretions, and the applications have been widened nowadays. Tumors that are located in the central airways can be treated with several bronchoscopic techniques, such as lasers (Nd:YAG), photodynamic therapy (PDT), brachytherapy, cryotherapy and electrocautery (argon, CO2), for palliation of airway obstruction in advanced cancers and for curative treatment of ROLCs. APC delivers a high-frequency alternating current to the tissue through an ionized argon gas in non-contact mode and has been extensively evaluated in open surgery of liver, spleen, and kidneys. From these evaluations it was shown that APC is an effective tool for superficial bleeding and controlling bleeding during gastrointestinal endoscopies4. In addition, it is attractive for the treatment of hemorrhagic superficial spreading tumor and also suitable for treating tumors 'around the corner' at a sharp angle. APC causes more acute superficial tissue destruction, thus, is more effective than any other method for the management of hemostasis5. However, it is less efficient for the in-depth tissue destruction of bulky tumors because it has a limited penetration depth of 2~3 mm. The cost of APC is less expensive and more readily available in many centers than lasers and photodynamic therapy (PDT). The possible complications are imminent respiratory failure, hemorrhage and life-threatening airway obstruction.
The results of previous studies showed that when electrocautery of rigid or flexible bronchoscopes for palliative treatment of lung cancer was used it was equally effective in achieving tumor coagulation and debulking compared to an Nd:YAG laser but with less excessive complication rates6-10. For icroinvasive lung cancer, PDT is preferentially used because of its strength of 'selective' damage where only relatively minor destruction occurs in normal tissue. However, any kinds of bronchoscopic technique can be effectively used when less than 3 millimeters of invasion of the bronchial mucosa and visible distal margin is required if the patient is not a candidate for surgery because of poor cardiopulmonary function11. The cases treated with electrocautery for occult cancer and typical intraluminal carcinoid were reported12.
Schuurman et al.13 reported a case of microinvasive and premalignant lesion detected by AFB and treated with APC and surgery. In addition, Kato et al.14 reported several cases where early lung cancers treated with PDT resulted in a reduction in the extent of required surgery. These reports clearly demonstrate the benefits of interventional bronchoscopy in that it provided an additional option of curative treatment to patient unable to tolerate surgery. In the present report, the patient was a candidate for pneumonectomy, with good preoperative physiologic function, but when on a mechanical ventilator in the operation room the patient was not able to tolerate one-lung ventilation and oxygen saturation dropped while the right upper lobectomy was performed. The pathology report of the in situ lesion revealed cancer cells of 1~2 millimeters' thickness and the basement membrane was intact. Therefore, we treated the in situ lesion with one session of APC, since the therapeutic depth of the diseased region was within the limits of APC. After APC treatment, the patient was free from immediate complications and from long-term sequelae, such as airway stenosis. Furthermore, there was no evidence of recurrence after 1 year. Consequently the patient was treated successfully with reduced-extent surgery by using APC as a curative treatment. We think interventional bronchoscopy, especially APC, is an effective, safe and economical tool for the curative treatment of ROLCs with superficial diseases, and it can be an alternative for patients who can't tolerate surgery.
Figures and Tables
Figure 1
Preoperative images of CT showed (A) about 3.7×2.7 cm sized heterogenous enhancing low density mass with total obstruction of anterior segmental bronchus of right upper lung (solid arrow), (B) proximal portion of right middle and lower bronchus is unremarkable (dashed arrow).
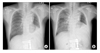
Figure 2
Initial bronchoscopy showed (A) intraluminal mass in RB3 (white dashed arrow) by conventional bronchoscopy, and (B) 'loss of autofluorescence' in superior segment of right lower bronchus (black solid arrow) by autofluorescence bronchoscopy. (C) APC was performed on the superior segment of right lower bronchus, (D) bronchoscopy immediate after APC showed debris, (E) and after two and half weeks ulcerative and mild edematous changes were shown. (F) No evidence of recurrence was shown on bronchoscopy on 3 months post-APC.
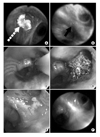
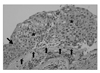
Figure 3
The report from pathologist revealed 'carcinoma in situ' for superior segment of right lower bronchus. The five small upward arrows shows intact basement membrane, and the region marked as '*' is filled with cancer cells with mitosis, pleomorphism. The downward arrow (left middle) revealed normal stratified squamous epithelium (H&E stain, ×200).
References
1. Lam S, MacAulay C, Hung J, LeRiche J, Profio AE, Palcic B. Detection of dysplasia and carcinoma in situ with a lung imaging fluorescence endoscope device. J Thorac Cardiovasc Surg. 1993. 105:1035–1040.
2. Pierard P, Vermylen P, Bosschaerts T, Roufosse C, Berghmans T, Sculier JP, et al. Synchronous roentgenographically occult lung carcinoma in patients with resectable primary lung cancer. Chest. 2000. 117:779–785.
3. Venmans BJ, van Boxem TJ, Smit EF, Postmus PE, Sutedja TG. Outcome of bronchial carcinoma in situ. Chest. 2000. 117:1572–1576.
4. Johanns W, Luis W, Janssen J, Kahl S, Greiner L. Argon plasma coagulation (APC) in gastroenterology: experimental and clinical experiences. Eur J Gastroenterol Hepatol. 1997. 9:581–587.
5. Freitag L. Interventional endoscopic treatment. Lung Cancer. 2004. 45:S235–S238.
6. Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001. 119:781–787.
7. Okada S, Yamauchi H, Ishimori S, Satoh S, Sugawara H, Tanaba Y. Endoscopic surgery with a flexible bronchoscope and argon plasma coagulation for tracheobronchial tumors. J Thorac Cardiovasc Surg. 2001. 121:180–182.
8. Stephens KE Jr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg. 2000. 119:289–296.
9. Hooper RG. Electrocautery in endobronchial therapy. Chest. 2000. 117:1820–1821.
10. Ledingham SJ, Goldstraw P. Diathermy resection and radioactive gold grains for palliation of obstruction due to recurrence of bronchial carcinoma after external irradiation. Thorax. 1989. 44:48–51.
11. Sutedja G, Schramel F, Postmus PE. Bronchoscopic treatment modalities in lung cancer, indications and limitations. Ann Oncol. 1995. 6:951–952.
12. Shah H, Garbe L, Nussbaum E, Dumon JF, Chiodera PL, Cavaliere S. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest. 1995. 107:1744–1751.
13. Schuurman B, Postmus PE, van Mourik JC, Risse EK, Sutedja TG. Combined use of autofluorescence bronchoscopy and argon plasma coagulation enables less extensive resection of radiographically occult lung cancer. Respiration. 2004. 71:410–411.
14. Kato H, Konaka C, Ono J, Kawate N, Nishimiya K, Shinohara H, et al. Preoperative laser photodynamic therapy in combination with operation in lung cancer. J Thorac Cardiovasc Surg. 1985. 90:420–429.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download