Abstract
We experienced a rare case of sinonasal sarcoidosis initially presenting as nasal cavitary mass. When the clinical course was different from that of typical tuberculosis, physician should think the possibility of sarcoidosis, and re-biopsy or retrospective review of pathological findings might be helpful.
In systemic sarcoidosis, the upper respiratory tract may be involved more frequently than generally believed1. However sinonasal involvement with sarcoidosis is not common. Nasal involvement is usually detected in patients in whom systemic sarcoidosis was previously diagnosed. In the absence of pulmonary disease, sinonasal sarcoidosis is rare2.
We report an unusual case of sarcoidosis in which the initial presentation was nasal cavitary mass without any sign of other organ involvement.
A 37-year-old woman presented to an out patient department of otolaryngology of our hospital with rhinorrhea and nasal obstruction for a year. She did not have any serious illness in past. Rhinologic examination revealed friable and swollen mucosa on both nasal cavity, massive hypertrophy of left inferior turbinate and septum (Figure 1). Nasal computed tomography (CT) demonstrated mucosal thickening of inferior turbinate and septum and soft tissue density in the ethmoid cavities bilaterally (Figure 2). The biopsy findings of left inferior turbinate and septum were non-caseating granuloma with necrosis (Figure 3). There was no remarkable involvement detectable with chest radiography.
Because the biopsy finding suggest granulomatous infection, tuberculosis of nasal cavity was suspected although microbiological evidence of tuberculosis was absent. She was prescribed antituberculous medication empirically with four drugs (isoniazid-rifampin-ethambutol-pyrazinamide). Follow-up otolaryngologic examination showed slight regression of mass on left inferior turbinate and there was slight improvement of her nasal symptom by four months medication.
After six months from the beginning of antituberculous medication, she complained pain of right second and fifth toe after long time driving. Foot radiography revealed the multiple fractures of her toes. The fractures were thought to stress fractures and she was splinted. She underwent a 99mTc-oxidronate (HDP) scintigraphy which revealed known fracture of toe and increased uptake on both sacroiliac joints and right navicular bone. Serum autoimmune antibodies, rheumatoid factor and HLA B27 was negative. These findings were considered to be suspicious for inflammatory arthritis. She was prescribed 7.5 mg methotrexate weekly and 7.5 mg prednisolone daily. After these medication, progressive improvement of her toe pain as well as nasal obstruction was seen. Methotrexate was maintained and prednisolone was tapered off. Three months later from the toe fracture, antituberculosis medication was finished for nine months and the nasal symptom was continued without significant change.
After a year from the patient's first visit to our hospital, the dryness of left eye was developed. Nodular mass on conjunctiva was seen from ophthalmologic examination and biopsied. This showed noncaseating granuloma with necrosis. Both cervical lymph nodes were palpated simultaneously. Serum angiotensin-converting enzyme was 59.5 U/L (normal <30 U/L). CT of thorax revealed multiple enlarged lymphadenopathies of supraclavicular area, mediastinum, both hilar area and both interlobar nodal areas (Figure 4A, B). These findings were seem to be consistent with sarcoidosis. She was treated with corticosteroids (prednisolone: 40 mg/day) for six months. With this treatment, her ophthalmologic, otolaryngologic and pulmonologic abnormalities were improved progressively.
Sarcoidosis is a multisystem immunologic disorder which has a predilection for pulmonary and upper respiratory tract involvement. Other sites of involvement include peripheral lymph nodes, liver, spleen, eyes, bones, skin, and lacrimal and salivary glands.
Sarcoidosis is rarely reported in Spain, Portugal, India, Saudi Arabia, South America, or far eastern countries because of the presence of other, more commonly recognized granulomatous diseases (tuberculosis, leprosy, fungal infection) that obscure sarcoidosis recognition3,4.
It is well known that non-caseating granulomas are not specific findings for the diagnosis of sarcoidosis. Because many infectious diseases can make granulomas, the need for microbiologic studies and cultures continues, especially when the patient has fever or when there are necrotic lesions in the biopsy specimens. Special stains for acid-fast bacilli and fungi are justified for the diagnosis, especially when there are atypical features for sarcoidosis such as necrosis or an air-space predominance of granulomas5,6.
Although any organ system can be involved by sarcoidosis, nasal involvement occurs in approximately 1% of cases of systemic sarcoidosis7. It is rare for sarcoidosis to affect the sinonasal tract exclusively2. Because symptoms and radiographic findings are similar to those of chronic sinusitis, which is far more common, sarcoidosis manifesting in the nasal cavity may have erroneously been underdiagnosed. Granulomatous sinonasal disease have a long list of differential diagnosis which are infectious rhinitis, Wegener's granulomatosis, polymorphic reticulosis, Churg-Strauss syndrome, lymphoma, berylliosis, or sarcoidosis8.
Braun et al9 proposed the following diagnostic criteria of sinonasal sarcoidosis: 1) histopathologic comfirmation of non-caseating granuloma; 2) chronic rhinosinusitis poorly responsive to conventional treatment and radiologic evidence of rhinosinusitis, often with nodules on the septum and/or the turbinates; 3) elevated level of angiotensin-converting enzyme; 4) positive gallium scan (if performed); 5) frequent evidence of systemic, especially pulmonary, sarcoidosis; 6) no evidence of other granulomatous diseases, such as Wegener' granulomatosis.
In our patient, the proper diagnosis was delayed for oneyear. The disease involved the eye, multiple lymph nodes, and lungs. Her fracture and swelling of toes were probably due to the sarcoidosis but those were not confirmed by biopsy. Several reasons exited for the delay of diagnosis of this uncommon disease. First, the initial presentation of the patient was the involvement of nasal cavity. Nasal involvement is usually detected in patients in whom systemic sarcoidosis was previously diagnosed. Second, the pathologic findings showed an atypical feature for sarcoidosis i.e. necrosis within the tissue. Those finding are more common in infectious disease such as tuberculosis6. The incidence of sarcoidosis was 0.13 person per 100,000 and of tuberculosis of any site was 73.0 persons per 100,000 in Korea10,11. So in the area of high prevalence of turberculosis, sarcoidosis is less likely to be concerned by clinician in the case of granulomatous disease.
Systemic corticosteroid has been long regarded the first line of treatment for both systemic and sinonasal sarcoidosis. Symptoms caused by sinonasal sarcoidosis can be effectively controlled with topical corticosteroid nasal sprays7. Surgery may have some role for sinonasal sarcoidosis but controversies remained12.
In conclusion, sinonasal sarcoidosis is uncommon and in the absence of any sign of other organ involvement, especially the lung, the diagnosis is quite difficult. Even with a biopsy, diagnosis of sarcoidosis is delayed because other granulomatous infections are more common.
Figures and Tables
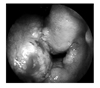 | Figure 1Endoscopic findings of nose shows massive hypertrophy of nasal septum and inferior turbinate. |
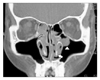 | Figure 2Coronal nasal CT shows soft tissue density (thin arrow) filling in both inferior ethmoid sinus, round soft tissue attenuated lesion (arrow head) in left maxillary sinus and lining mucosal thickening (thick arrow) of nasal septum and inferior turbinate. |
References
1. Zeitlin JF, Tami TA, Baughman R, Winget D. Nasal and sinus manifestations of sarcoidosis. Am J Rhinol. 2000. 14:157–161.
2. Tay HL, Vaughan-Jones R, Qureshi SS. Ethmoidal sarcoidosis. J Laryngol Otol. 1994. 108:682–684.
3. Mana J, Badrinas F, Morera J, Fite E, Manresa F, Fernandez-Nogues F. Sarcoidosis in Spain. Sarcoidosis. 1992. 9:118–122.
4. James GD. Sarcoidosis and other granulomatous disorders. 1994. 1st ed. New York: Marcel Dekker.
5. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999. 160:736–755.
6. Sheffield EA, Williams WJ. James DG, editor. Pathology. Sarcoidosis and other granulomatous disorders. 1994. New York: Marcel Dekker;45–67.
7. McCaffrey TV, McDonald TJ. Sarcoidosis of the nose and paranasal sinuses. Laryngoscope. 1983. 93:1281–1284.
8. Krespi YP, Kuriloff DB, Aner M. Sarcoidosis of the sinonasal tract: a new staging system. Otolaryngol Head Neck Surg. 1995. 112:221–227.
9. Braun JJ, Gentine A, Pauli G. Sinonasal sarcoidosis: review and report of fifteen cases. Laryngoscope. 2004. 114:1960–1963.
10. Kim DS. Sarcoidosis in Korea: report of the Second Nationwide Survey. SarcoidosisVasc Diffuse Lung Dis. 2001. 18:176–180.
11. Domestic Statistics "Trend of case notification rate per 100,000 by year, 2001-2005". The Korean National Tuberculosis Association. 16 Jul. 2007. Available from:
http://www.knta.or.kr/korea/study/study05.asp.
12. Marks SC, Goodman RS. Surgical management of nasal and sinus sarcoidosis. Otolaryngol Head Neck Surg. 1998. 118:856–858.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download