Gefitinib is an oral selective inhibitor that targets on tyrosine kinase of the epidermal growth factor receptor. The prevalence of interstitial lung disease as a adverse consequence of gefitinib therapy is 2% in the Japanese and 0.3% in Americans1,2; one third of these patients are fatalities. The clinical characteristics that predict adverse pulmonary effects are known to be being a male patient and the presence of a smoking history1,3. Those patients with a low probability of EGFR mutation have a low possibility of achieving a beneficial effect from gefitinib. In accord with this, none of the patients in a retrospective study who harbored EGFR mutation were shown to develop any pulmonary complications4. Several series5-13 have reported on fatal respiratory complications related to gefitinib in patients with non-small cell lung cancer, but any information on EGFR mutation in these patients was not available.
We described here a woman who never smoked and who suffered with adenocarcinoma of the lung and she had EGFR mutation. She developed acute fatal respiratory failure after treatment with gefitinib.
A 47-year-old woman was admitted with complains of cough, sputum and progressive dyspnea. She was diagnosed with adenocarcinoma of the lung on biopsy of a supraclavicular lymph node and the sputum cytology. On her chest CT scan, a 4×2.6 cm sized mass was noted at the superior segment of the left lower lobe and there were multiple nodules scattered in both lung (Figure 1). In addition, bone metastasis to the ileum and acetabulum was found on positron emission tomography. She was finally staged as cT4N3M1.
She was a never-smoker and she denied a history of medical disease, including cardiovascular, allergic, rheumatologic or respiratory diseases. Her ECOG performance status was four because of her severe respiratory distress (Borg scale 8). At the time of admission, her body temperature was 36.8℃ and the blood pressure was 140/70 mmHg. Rhonchi were audible in both lungs. With administering oxygen supplementation via a nasal cannula at a flow rate of 5 L/min, the arterial blood gas study revealed a pH of 7.44, a PaO2 of 74.0 mmHg and a PaCO2 of 35.1 mmHg. The laboratory findings showed a leukocyte count of 6,200/mm3 (78.4% neutrophils, 13.7% lymphocytes, 3.7% monocytes, 1.7% eosinophils, and 1.1% basophils), a hemoglobin level of 14.5 g/dl and a hematocrit of 45.3%. The serum lactate dehydrogenase level was 361 IU/L, and the C-reactive protein level was 5.56 mg/dl. The results of the hepatic and renal function testing were normal. Azithromycin and 2nd generation cephalosporin was given empirically until the histologic diagnosis was finally made. Her respiratory distress was not improved in spite of administering oral bronchodilators, β-2 agoninst nebulization and nasal oxygen. EGFR mutation was analyzed on the biopsy specimen of the lymph node by performing DNA sequencing of exons 18~21 in the EGFR tyrosine kinase domain, and the in-frame deletion mutation in codon 746~750 of exon 19 was demonstrated (Figure 2).
Oral gefitinib (IRESSA®), 250 mg once daily was given as the first line treatment on September, 24th 2006. Her dyspnea began to significantly improve to four on the Borg scale on the 3rd day of gefitinib administration, along with improvement being noted on the chest radiography. She was taken off nasal oxygen supplementation on day 7. On day 16, severe dyspnea suddenly developed along with a high fever (38.8℃). Diffuse bilateral ground-glass opacities were noted on the chest CT scan, while the multiple lung nodules and a lung mass at the superior segment of the left lower lobe were all decreased in size. The antibody test for Mycoplasma pneumoniae or antigen tests for Legionella and Pneumococus were negative. No bacterial pathogens, fungus or virus were identified in the cultures from the sputum or blood. Although high dose corticosteroid was immediately administrated under a strong clinical suspicion of drug-induced, acute interstitial pneumonitis, she rapidly deteriorated; sadly, she died of respiratory failure on day 17. Her family did not want a postmortem examination.
We report here on a case of acute fatal respiratory failure in a lung cancer patient who had the characteristics favoring a good response to gefitinib: a woman, a never-smoker and she had adenocarcinoma and EGFR mutation. It could be suggested that clinicians should use gefitinib with caution even if the patient displays this favorable phenotype for which gefitinib is regarded as safe to use. Although this case has a caveat because no postmortem examination was performed to differentiate between the other diagnoses, the only way to confirm the diagnosis of drug induced lung injury is to rechallenge with the suspected drug, and that is not possible for lung cancer patients.
Ethnic differences in the genetic susceptibility and the role of the EGFR gene have been suggested as hypothetical mechanisms of gefitinib-induced lung disease. As for the role of EGFR in gefitinib-induced lung injury, a study conducted with a murine model showed that gefitinib augmented the pulmonary fibrosis induced by bleomycin, and the effect of gefitinib was demonstrated to occur through inhibiting EGFR phosphorylation14. This could be supportive evidence for our present case. On the contrary, another study that had a similar design, but it used three differential doses of gefitinib, showed its preventive effect on pulmonary fibrosis15. In addition, a recent retrospective analysis by Fujiwara et al4 showed that all eleven patients with EGFR mutation didn't demonstrate any pulmonary toxicity with receiving gefitinib therapy. Although the results from clinical studies1,3 have classified males, never-smokers and non-adenocarcinoma patients as the risk group for developing adverse respiratory effects, how the somatic or genomic mutation of the EGFR gene affects the mechanism of gefitinib induced lung injury is still unknown, and this will require future study.
Figures and Tables
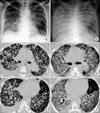 | Figure 1At the time of diagnosis, bilateral multiple lung nodules, right pleural effusion and a mass at the superior segment of the left lower lobe were observed on chest PA (A) and chest CT scans (B, C). At the 17th day after gefitinib was administered, the multiple lung nodules, pleural effusion and lung mass were shown to be decreased in size on the chest AP (D) and on similar slices from the pulmonary embolus protocol CT scan (E, F) while diffuse ground glass opacities were newly developed in both lungs. |
References
1. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.
2. Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004. 10:1212–1218.
3. Takano T, Ohe Y, Kusumoto M, Tateishi U, Yamamoto S, Nokihara H, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004. 45:93–104.
4. Fujiwara Y, Kiura K, Toyooka S, Takigawa N, Tokumo M, Hotta K, et al. Relationship between epidermal growth factor receptor gene mutations and the severity of adverse events by gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2006. 52:99–103.
5. Seto T, Seki N, Uematsu K, Tanigaki T, Shioya S, Koboyashi T, et al. Gefitinib-induced lung injury successfully treated with high-dose corticosteroids. Respirology. 2006. 11:113–116.
6. Ohyanagi F, Ando Y, Nagashima F, Narabayashi M, Sasaki Y. Acute gefitinib-induced pneumonitis. Int J Clin Oncol. 2004. 9:406–409.
7. Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003. 361:137–139.
8. Rabinowits G, Herchenhorn D, Rabinowits M, Weatge D, Torres W. Fatal pulmonary toxicity in a patient treated with gefitinib for non-small cell lung cancer after previous hemolytic-uremic syndrome due to gemcitabine. Anticancer Drugs. 2003. 14:665–668.
9. Ieki R, Saitoh E, Shibuya M. Acute lung injury as a possible adverse drug reaction related to gefitinib. Eur Respir J. 2003. 22:179–181.
10. Okamoto I, Fujii K, Matsumoto M, Terasaki Y, Kihara N, Kohrogi H, et al. Diffuse alveolar damage after ZD1839 therapy in a patient with non-small cell lung cancer. Lung Cancer. 2003. 40:339–342.
11. Inomata S, Takahashi H, Nagata M, Yamada G, Shiratori M, Tanaka H, et al. Acute lung injury as an adverse event of gefitinib. Anticancer Drugs. 2004. 15:461–467.
12. Nagaria NC, Cogswell J, Choe JK, Kasimis B. Side effects and good effects from new chemotherapeutic agents. Case 1. Gefitinib-induced interstitial fibrosis. J Clin Oncol. 2005. 23:2423–2424.
13. Sumpter K, Harper-Wynne C, O'Brien M, Congleton J. Severe acute interstitial pnuemonia and gefitinib. Lung Cancer. 2004. 43:367–368.
14. Suzuki H, Aoshiba K, Yokohori N, Nagai A. Epidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosis. Cancer Res. 2003. 63:5054–5059.
15. Ishii Y, Fujimoto S, Fukuda T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am J Respir Crit Care Med. 2006. 174:550–556.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download