Abstract
A unique case of atypical pulmonary sarcoidosis in a 62-year-old man complaining of dyspnea is presented. Chest CT scan showed an unusual pattern and distribution of pulmonary sarcoidosis manifesting mainly as reticular densities, interlobular septal thickening, and ground-glass opacities, in the subpleural and lower lung predominancy. However, a surgical lung biopsy revealed classical findings of sarcoidosis. Knowledge of this atypical pulmonary involvement may improve understanding sarcoidosis as the great masquerader.
Sarcoidosis is a systemic granulomatous disease of unknown etiology and can affect any organ system. The thorax is one of the most commonly affected areas, and thoracic involvement is reported in up to 90% of patients with sarcoidosis1-4. Thoracic sarcoidosis usually affects the lymph nodes and the lung parenchyma. Radiologic manifestation of thoracic sarcoidosis is well established, and symmetric bilateral hilar and mediastinal lymphadenopathy with or without concomitant lung parenchymal infiltrates is the most common manifestation of thoracic sarcoidosis1,3. Although 60% to 70% of patients with thoracic sarcoidosis have characteristic radiologic findings, the remaining patients may have a variety of radiologic findings5,6. Furthermore, the appearance of pulmonary sarcoidosis is occasionally very unusual, nonspecific, and atypical. Therefore, pulmonary sarcoidosis often resembles many other lung diseases.
A rare, atypical case of thoracic sarcoidosis manifesting with an unusual pattern and distribution of lung involvement is reported in this paper.
A 62-year-old man visited the hospital with a complaint of NIHA class II dyspnea. On auscultation, his breathing sound was normal and on a thorough physical examination, no abnormalities were found. The patient had a past history of hypertension and was an ex-smoker of 40-pack years. In laboratory tests, collagen vascular disease markers were negative.
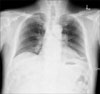
Simple chest radiography was performed as a routine check-up. Lymph node enlargement was suspected in both hilum (Figure 1). A chest CT scan taken 4 days later showed fine reticular densities, interlobular septal thickenings, and faint ground-glass opacities on both lungs. The lesions were distributed on both lungs symmetrically, but predominant lower and subpleural lung involvement was noted (Figure 2A, B). Discrete lymph node enlargements without internal calcifications were observed in the prevascular, subaortic, subcarinal, and both paratracheal and hilar areas (Figure 2C). Thus, with the presumptive diagnosis of idiopathic interstitial pneumonia such as nonspecific interstitial pneumonitis and lymphocytic interstitial pneumonia, or lymphoma, a surgical lung biopsy was performed on the left upper and lower lobes. A mediastinoscopic biopsy was also performed on the right paratracheal lymph nodes. However, the result of the lung biopsy revealed extensive infiltration by noncaseating granulomas distributed along the pleura, interlobular septa, and bronchovascular bundles, which are characteristic findings of pulmonary sarcoidosis (Figure 3). A mediastinal lymph node biopsy also revealed noncaseating chronic granulomatous inflammation consisting of sarcoidosis.
The CT features of pulmonary sarcoidosis include micronodules and nodules, central peribronchovascular thickening, alveolar or pseudoalveolar consolidations, septal and nonseptal lines, ground-glass opacity, conglomerate masses, lung architectural distortion, bronchiectasis, honeycombing or other types of cyst, emphysema, and thickening of the pleural surface1. Pulmonary sarcoidosis is typically bilateral and symmetric, and involves mainly the central rather than peripheral lungs. The upper lobes, particularly the apical and posterior segments, are the most severely affected2.
The most characteristic CT appearance of pulmonary sarcoidosis consists of small nodules in a perilymphatic distribution1,2,6. Small nodules usually have irregular margins and range from 1 to 10 mm in size. They are typically scattered throughout the interstitium of the lungs, along the lymphatics, particularly in the peribronchovascular and subpleural spaces, and along the interlobular septa. Pathologically, noncaseating granulomas distribute mostly along the lymphatics in the peribronchovascular sheath and, to a lesser extent, in the subpleural and interlobular septal lymphatics6,7. However, the diseases in lymphatic and perilymphatic distribution include lymphangitic carcinomatosis, lymphoproliferative disease, silicosis, and amyloidosis as well as sarcoidosis. Therefore, CT findings of these diseases can be similar to that of sarcoidosis3,8. Sarcoidosis is more significantly involved bilaterally and in the upper lungs than lymphangitic carcinomatosis and malignant lymphoma. Thickened interlobular septa observed in lymphangitic carcinomatosis are rare in sarcoidosis8. Lymphoma may present with predominant peribronchovascular nodules but are typically larger and less profuse than sarcoidosis. Silicosis also shows perilymphatic nodules, but the distinction can be made easily by clinical history. Thus, sarcoidosis can usually be differentiated from these diseases.
Sarcoid granulomas can coalesce and become confluent to produce large nodules and alveolar opacities. The alveolar opacities vary in size from 1~10 cm, are bilateral with ill-defined borders, and may show internal air bronchograms. This peripheral nonsegmental airspace consolidation is called alveolar sarcoidosis. Pulmonary sarcoidosis sometimes shows patchy areas of ground-glass opacity, which may be superimposed on a background of interstitial nodules or fibrosis. Other CT findings include solitary or multiple pulmonary masses, atelectasis, focal or asymmetric alveolar or interstitial disease. Pulmonary sarcoidosis can show miliary nodules located in a random distribution. This miliary pattern can show similar findings of miliary tuberculosis, miliary fungal infection, and hematogenous metastasis.
Pulmonary sarcoidosis may resolve spontaneously or progress to fibrosis. As fibrosis progresses, irregular reticular densities, lung parenchymal distortions with large conglomerate mass, and lung volume loss are predominant. These findings can be associated with traction bronchiectasis and peripheral honeycombing2.
In the present case, fine reticular densities and interlobular septal thickening with basal subpleural distribution, and faint ground-glass opacity without a background of visible interstitial nodules rendered a diagnosis of sarcoidosis difficult. Furthermore, areas of ground-glass attenuation and reticular opacities distributed peripherally in both lower lung zones were contrary to the upper and central lung distribution pattern of classic pulmonary sarcoidosis. These are the dominant CT findings among patients with nonspecific interstitial pneumonia, and they are similar to lymphocytic interstitial pneumonia, which manifests with diffuse ground-glass attenuation, poorly defined centrilobular nodules, thickening of perilymphatic interstitium, septal thickening, and scattered thin-walled cysts with lower lung zone predominance9. Hence, an initial diagnosis was made of idiopathic interstitial pneumonia, such as nonspecific interstitial pneumonia and lymphocytic interstitial pneumonia, rather than sarcoidosis in this case. Therefore, a surgical lung biopsy was performed in the left upper and lower lobes to make a diagnosis. However, histologically, the pathologic specimen showed classical findings of pulmonary sarcoidosis.
In this report, a rare atypical case of pulmonary sarcoidosis manifesting mainly as reticular densities, interlobular septal thickening, with ground-glass opacities, in subpleural and lower lung predominancy is presented. Pulmonary sarcoidosis can mimic other lung diseases radiologically and cause radiologic diagnostic uncertainties. Awareness of the diverse manifestations as well as typical manifestations can help prevent unnecessary procedures in patients with sarcoidosis.
Figures and Tables
 | Figure 2Thin-section CT with lung window setting (A, B) shows fine reticular densities, faint ground-glass opacities, and interlobular septal thickenings on both lungs, predominantly the lower subpleural lung zone. Enhanced chest CT scan with a mediastinal window setting (C) shows bilateral mediastinal and hilar lymphadenopathies. |
References
1. Nunes H, Brillet PY, Valeyre D, Brauner MW, Wells AU. Imaging in sarcoidosis. Semin Respir Crit Care Med. 2007. 28:102–120.
2. Vagal AS, Shipley R, Meyer CA. Radiological manifestations of sarcoidosis. Clin Dermatol. 2007. 25:312–325.
3. Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004. 24:87–104.
4. Hennebicque AS, Nunes H, Brillet PY, Moulahi H, Valeyre D, Brauner MW. CT findings in severe thoracic sarcoidosis. Eur Radiol. 2005. 15:23–30.
5. Hamper UM, Fishman EK, Khouri NF, Johns CJ, Wang KP, Siegelman SS. Typical and atypical CT manifestations of pulmonary sarcoidosis. J Comput Assist Tomogr. 1986. 10:928–936.
6. Müller NL, Kullnig P, Miller RR. The CT findings of pulmonary sarcoidosis: analysis of 25 patients. AJR Am J Roentgenol. 1989. 152:1179–1182.
7. Nishimura K, Itoh H, Kitaichi M, Nagai S, Izumi T. Pulmonary sarcoidosis: correlation of CT and histopathologic findings. Radiology. 1993. 189:105–109.
8. Honda O, Johkoh T, Ichikado K, Yoshida S, Mihara N, Higashi M, et al. Comparison of high resolution CT findings of sarcoidosis, lymphoma, and lymphangitic carcinoma: is there any difference of involved interstitium? J Comput Assist Tomogr. 1999. 23:374–379.
9. Johkoh T, Müller NL, Pickford HA, Hartman TE, Ichikado K, Akira M, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999. 212:567–572.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download