Abstract
Tracheal tuberculosis is a relatively uncommon form of tuberculosis and can result in acute tracheal obstruction early in the disease course. This is a report of a case of tracheal tuberculosis with unusual clinical presentation.
Endobronchial tuberculosis (EBTB) is a tuberculous infection of the tracheobronchial tree and can be diagnosed by microbial and histopathological examination1. Endotracheal tuberculosis is a relatively uncommon (about 4%), localized form of EBTB which may cause acute respiratory failure2. Early diagnosis and prompt treatment, before the development of fibrotic scarring, is important to prevent tracheobronchial stenosis. But the clinical, radiologic presentation of EBTB is not specific, and early diagnosis of EBTB depends on a high index of physician's suspicion3. We report a case of endotracheal tuberculosis who presented with acute tracheal obstruction early in the course of the disease; this was resolved by bronchoscopic intervention without tracheal stricture.
A 23-year-old married woman presented with a two month history of cough, sputum and voice change. She was relatively healthy and had no past medical history of note or drug history. She had never been a smoker. Laboratory findings were normal except the sputum smear was positive for acid-fast bacilli (AFB). A chest radiograph showed no abnormality. She was referred to us because her symptoms continued despite treatment with an anti-tuberculosis drug for two weeks. On physical examination, breathing sound was clear, without wheezing. To rule out the possibility of EBTB, we performed a fiberoptic bronchoscopy. A large caseous mass was found on the right lateral wall of the distal trachea (Figure 1A). A bronchial biopsy revealed caseous necrosis with epithelioid granuloma; a bronchial washing sample showed culture positive for AFB. Prednisolon was additionally prescribed in a dose of 40 mg daily with an anti-tuberculosis drug. Two weeks later, the patient revisited the office with a severe cough and dyspnea which had developed acutely. She complained that something was moving up and down inside her neck but she could not expectorate it. On physical examination, expiratory wheezing was heard on her neck. A pulmonary-function test (PFT) demonstrated that forced expiratory volume in one second (FEV1) was 0.62 L (predicted 3.44 L) and forced vital capacity (FVC) was 1.75 L (predicted 3.76 L). The flow-volume curve showed the typical pattern of a variable intrathoracic airway obstruction (Figure 2A). A second bronchoscopy showed that the mass was flopping up and down with expiration and inspiration, respectively. During expiration, the mass moved up to mid-trachea level and obstructed the tracheal lumen. Moreover, the mid-tracheal lumen was narrowed with mucosal swelling and positive pleural pressure during expiration (Figure 1B). The mass moved down to the distal trachea (Figure 1C). Mass was removed by biopsy forceps without significant bleeding. Immediately after the removal, the cough and dyspnea dramatically improved. PFT improved remarkably as follows; FEV1; 2.32 L (predicted 3.44 L), FVC; 2.83 L (predicted 3.76 L). The flat expiratory limb of flow volume curve changed to the normal pattern (Figure 2B). The removed mass was round, smooth and had a fibrous tag (Figure 2C). Most portions of the tissue were necrotic, and numerous AFB were noted on microscopic examination (Figure 2D). On the superficial portion of the mass, microscopy revealed septate hyphae with acute angle branching (Figure 2E). The patient was treated with antituberculosis chemotherapy for nine months. Nine months after the treatment, a third bronchoscopy was performed. Symptoms of active EBTB had completely disappeared, but a round crater like depression with the loss of several tracheal cartilage rings was observed on the distal of the trachea (Figure 1D). Two years later, a follow up bronchoscopy revealed no evidence of recurrence or tracheal stricture.
EBTB has been reported to develop in 10~40% of patients with active pulmonary TB3. It is a disease of young patients, with more than 50% of cases being observed at less than 35 years of age. Peak incidence of EBTB occurs in the third decade, with a female preponderance4. In spite of advances in the diagnosis and management of endobronchial tuberculosis, bronchial stricture still remains a considerable clinical problem5,6. Bronchial stricture can be associated with 60~95% of EBTB patients2 but is usually a late and severe complication of EBTB4. However, our patient, presented acute upper airway obstruction early in the clinical course, which is different from the usual clinical course of EBTB. It is difficult to explain how an initial caseous endotracheal mass changed into a polyp-like lesion. One of the possible explanations is that an enlarged tracheal lymph node eroded into the trachea, and fibrous tissue, attached to the mass, acted like a stalk. But the removed polyp-like mass showed necrotic change and did not reveal features of a lymph node. Moreover, the initial chest CT did not show significant enlargement of the paratracheal lymph nodes around the trachea. Even though the mucosal swelling of the trachea was severe on the first two bronchoscopies, tracheal stenosis did not occurr. In this case, the tuberculosis infection involved several tracheal cartilages but, fortunately destruction was limited to the lateral part of the tracheal cartilage rings. The bronchoscopy at the ninth month and two years after the treatment showed a crater like depression on the distal trachea with loss of the lateral part of several tracheal cartilages. If the tuberculosis had involved all the tracheal cartilage rings, it would have been problematic due to tracheal stricture. In our previous case report7, we observed that even though one-third of several tracheal cartilages had been lost in the endotracheal tuberculosis, significant tracheal stricture did not occur. The remaining two-thirds of the tracheal cartilage rings seemed to be strong enough to support the tracheal lumen opening during the respiratory cycle. The degree of tracheal cartilage involvement would be an important factor in the development of tracheal stricture in patients with endotracheal tuberculosis. Also, we cannot explain why aspergillosis hypae was observed incidentally in the superficial portion of the mass. It may be possible that saprophytic colonization occurred on the surface. The patient showed excellent clinical results without any specific treatment for aspergillosis.
Clinical manifestations of endobronchial tuberculosis include a chronic productive cough, barking cough, hemoptysis, chest pain, general weakness, fever, dyspnea4, and expectoration of bronchial cartilage7. EBTB can mimic bronchogenic carcinoma8, polypoid mass9, asthma10, foreign body aspiration, pneumonia and atelectasis4. Such various clinical features are a frequent cause of diagnostic delay. Therefore, a high index of physician's suspicion is important for early diagnosis and effective treatment3. Diagnosis and follow up of EBTB mainly depends on bronchoscopy2,8,11. It has been reported that during the initial three months, the therapeutic outcome of EBTB can be predicted. But it is difficult to predict the outcome of a tumorous type of EBTB because the evolution of lesions during treatment is complicated, and bronchial stenosis develops frequently12. Our patient, who had tumorous type of EBTB, showed severe symptomatic changes during the first two weeks of treatment. Therefore, it is important to monitor the therapeutic response carefully by observing symptomatic changes and prescribing a follow-up bronchoscopy especially during initial treatment periods.
Figures and Tables
Figure 1
Serial findings of bronchoscopy. The first bronchoscopy (A) shows a caseous protruding mass-like lesion on the right lateral wall of the distal trachea, and the tracheal lumen is narrowed by severe mucosal swelling. Two weeks later, a second bronchoscopy shows up (B) and down (C) movement of a polyplike mass during expiration and inspiration, respectively. Nine months later, a third bronchoscopy (D) shows a crater-like depression on the distal trachea. Several lateral parts of the tracheal cartilage rings have disappeared and the tracheal cartilage rings are clearly visible due to improvement of the mucosal swelling.
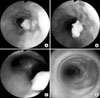
Figure 2
Findings of flow volume curve before (A) and after (B) mass removal Typical variable intrathoracic airway obstruction is observed (A). After mass removal, a flat expiratory limb of flow volume curve is changed to the normal pattern (B). Gross specimens (C) of removed polyplike mass show AFB (D) in the central portion of the mass and branching hypae in the surface of the mass (E) (AFB stain, ×400, H&E stain, ×400).

References
1. Hoheisel G, Chan BK, Chan CH, Chan KS, Teschler H, Costabel U. Endobronchial tuberculosis: diagnostic features and therapeutic outcome. Respir Med. 1994. 88:593–597.
2. Morrone N, Abe N. Bronchoscopic findings in patients with pulmonary tuberculosis. J Bronchol. 2007. 14:15–18.
3. Ip MS, So SY, Lam WK, Mok CK. Endobronchial tuberculosis revisited. Chest. 1986. 89:727–730.
4. Lee JH, Park SS, Lee DH, Shin DH, Yang SC, Yoo BM. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest. 1992. 102:990–994.
5. Watanabe Y, Murakami S, Iwa T. Bronchial stricture due to endobronchial tuberculosis. Thorac Cardiovasc Surg. 1988. 36:27–32.
6. Han JK, Im JG, Park JH, Han MC, Kim YW, Shim YS. Bronchial stenosis due to endobronchial tuberculosis: successful treatment with self-expanding metallic stent. AJR Am J Roentgenol. 1992. 159:971–972.
7. Park MJ, Woo IS, Son JW, Lee SJ, Kim DG, Mo EK, et al. Endobronchial tuberculosis with expectoration of tracheal cartilages. Eur Respir J. 2000. 15:800–802.
8. Smith LS, Schillaci RF, Sarlin RF. Endobronchial tuberculosis. Serial fiberoptic bronchoscopy and natural history. Chest. 1987. 91:644–647.
9. Ohno S, Matsuoka R, Mieno T. A case of traceal tuberculosis showing polypoid proliferation in the trachea (Japanese). J Jpn Soc Bronchol. 1987. 9:254–258.
10. Park CS, Kim KU, Lee SM, Jeong SW, Uh S, Kim HT, et al. Bronchial hyperreactivity in patients with endobronchial tuberculosis. Respir Med. 1995. 89:419–422.
11. Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000. 117:385–392.
12. Lee JH, Chung HS. Bronchoscopic, radiologic and pulmonary function evaluation of endobronchial tuberculosis. Respirology. 2000. 5:411–417.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download