Abstract
Background
In pathogenesis and prognosis of lung cancer, significance of enormous types of genetic expression were very compounding and undetermined. We performed this study to search association between clinical characteristics and expression of COX-2, MMP-9 and p53 in non-small cell lung cancer.
Methods
Ninety-one patients with adenocarcinoma or squamous cell carcinoma were enrolled. We had searched clinical data retrospectively and performed immunohistochemical staining for COX-2, MMP-9 and p53. We had analyzed significance of these three genes in clinical features and prognosis for survival.
Results
1) In squamous cell carcinoma, male was predominant and was significantly correlated with smoking. 2) Major prognostic determinants for overall survival were curative resection. 3) Expression of COX-2 was more frequent in adenocarcinoma than in squamous cell carcinoma. 4) Negative staining of COX-2, MMP-9 and p53 was more frequent in squamous cell carcinoma than adenocarcinoma. 5) Survival duration was longer in the group with positive expression of p53 and negative for COX-2 and MMP-9 (median duration of survival = 165.6 weeks) than groups with the other expressional patterns. 6) Significant correlation was found between expression of MMP-9 and COX-2. In squamous cell carcinoma, expression of MMP-9, COX-2 and mutant p53 were mutually correlated. 7) COX-2 expression was significant prognostic factor for survival in resected cancer group. In unresected inoperable non-small cell lung cancer group, MMP-9 was statistically significant prognostic factor for overall survival.
Lung cancer is the leading type of cancer and the leading cause of mortality from cancer -i.e., 17.8% of all cancer deaths worldwide1. A variety of oncobiologic studies have been focusing on oncogenes and tumor suppressor genes and enormous genes related to cell cycle control and apoptosis. So far, the mechanism of development and progression of non-small cell lung cancer (NSCLC) was explained firstly as multistep, multifocal and yet clonal processes by chromosomal instabilities2-6, allelic losses and microsatellite instabilities7-12. Another hypothesis for carcinogenesis is the field cancerization by which tobacco smoke and its 60 carcinogens cause the whole bronchial epitleial cells to be exposed to increased risk of developing lung cancer13-15. In recent data, many other genes related to inflammation, extracellular matrix also be concerned in tumorigenensis. More defined knowledge of lung carcinogenesis must be ascertained and exploited to enhance survival rates of patients suffering from lung cancer.
In this study, we evaluated expression of Cyclooxygenase-2(COX-2), Matrix Metalloproteinase-9(MMP-9), p53 in NSCLC and analyzed prognostic significance.
COX-2 is induced by inflammatory stimuli. COX-2 over-expression has been found in many solid tumors-hepatocellular carcinoma16, squamous cell carcinoma of esophagus17, squamous cell carcinoma of skin18, colorectal adenocarcinoma19-21.
There is evidence that cyclooxygenase-2 (COX-2) is overexpressed in lung cancer and promotes tumor proliferation, invasion, angiogenesis, and resistance to apoptosis22.
Wild type p53 inhibits G1 to S transition through induction of p21 and facilitating apoptosis and DNA repair through BAX induction and DNA repair enzymes23-25. The mechanism of activation in this protein comes from modification of the protein rather than genetic transcriptional or translational upregulation26-29. Mutant type p53 expression has been consistently associated shorter survival but some studies have failed to fine such results30,31.
Recently, several matrix metalloproteinases have shown promise as prognosticators in non-small cell lung cancer. Excessive or inappropriate expression of matrix metalloproteinase may contribute to the pathogenesis of tissue destructive processes in a wide variety of diseases including lung diseases. MMP-9 is known to have some important role for metastasis in various cancers.
It is necessary to determine the clinical significance of these three genes in NSCLC. Genetic mechanisms for cancer development were very complicated and main schemata were concentrated on tumor suppressor genes and oncogenes and genes related cell cycle and apoptosis.
Co-expression of carcinogenic genes was more meaningful than a single as biological predicators on survival rate of NSCLC, it is worth performing further studies.
COX-2 is related to tumor proliferation and progression, MMP-9 is important in interaction between tumor cells and adjacent interstitium and metastasis. Some studies had reported data about prognostic implication and correlation among p53, MMP-9 and COX-2 but these results are inconclusive. Some immunohistochemical findings question a relationship between COX-2 and p53 as a marker of malignancy in colorectal cancer tissues32. An immunohistochemical study of human colon carcinomas designed to look for coexpression of MMP and COX-2 revealed that 80% of the specimens were overexpressed both COX-2 and matrilysin in the neoplastic epithelium33.
In NSCLC, prognosis in overall survival can be affected by expression of COX-2, MMP-9 and p53.
We intended to determine whether expression of COX-2, MMP-9 and mutant p53 could contribute to prognosis in overall survival in patients with NSCLC.
We retrospectively searched clinical data based on clinical charts and radiologic films of 91 patients with non-small cell lung cancer diagnosed and treated in Chung-Ang University hospital from 1990 to 2004. Among clinical data, we could not definitely estimate clinical performance status. We performed clinical or pathologic staging of cancers based on the TNM criteria of American Joint Committee for Cancer 2001. Eligibility criteria were that were thatthe study patients should be those with pathologically confirmed non-small cell lung cancer as squamous cell carcinoma and adenocarcinoma. Two histologic types could be enrolled due to eligible statistical analysis. Exclusion criteria were other histologic types of NSCLC, which was lesser numbers for statistical analysis and patients who could not be followed up.
With 4 - 5 µm-thick paraffin-embedded tissues of pulmonary carcinoma, we performed deparaffinization and soaking them into 90%, 75% and 50% alcohol solution for 2 minutes. Next, those tissues were treated with 0.3% hydrogen peroxidemethanol for 10 minutes and washed with distilled water and sequentially with 50 mM Tris-buffered soultion(TBS, pH 7.5). Then those were treated with goat serum for 30 minutes. After removal of remnant solution, those were incubated with primary human antibodies for COX-2(1:50, Zymed, South San Francisco, CA, USA), MMP-9(1:50, Zymed,South San Francisco, CA, USA), p53 (1:50, Zymed, South San Francisco, CA, USA) for 2 hours in room temperature. Subsequently, these were washed three times with TBS. As a next procedure, secondary antibody labeled with biotin (Streptavidin biotin kit, Zymed, South San Francisco, CA, USA) was applied to them for 20 minutes and then stained with conventional Avidin-Biotin Complex method. For development, 3-amino-9-ethylcarbazole(AEC) was used. For contrast staining, Mayer's hematoxylin was used. And staining results were reported with light microscopy.
As for COX-2 and MMP-9 expression, positive result was defined if tumor cells with intracytoplasmic or membranous staining were more than 10 percent in overall field. Mutant p53 expression was defined as positive if tumor cells with intranuclear staining were more than 10% among total carcinoma cells in the whole field under light microscopy.
Statistical analyses were performed with the use of statistical software package, SPSS (version 11.5). Between two groups, differences of frequency were compared with chi-square test. For comparing the mean between two groups, unpaired T test was used. Correlation analysis was done using Pearson's correlation analysis. Survival analysis was performed with Kaplan-Meier method and Log rank test. To define significant prognostic factors for overall survival, Cox's proportional hazards model was applied. In all statistical test in this study, significance was determined if p value was less than 0.05.
In 91 cases, proportion of male patients was 81.3%. In squamous cell carcinoma in the lungs, proportion of male was predominant than that of female(p=0.000). Proportion of smokers was also significantly higher than that in adenocarcinoma (p=0.001). Smoking history was higher in men than women (p=0.000) (Table 2).
Thirty four cases (37.4%) had early stage (stage I, II) of NSCLC, so cases with NSCLC in advanced stage(stage III, IV) were double to early ones. Curative resection of the lung was performed in 41.8%. In the end of study, 71.4% of patients were dece ased and the others were censored. Median duration of survival in all the enrolled patients was 62.7 weeks.
Expressional rates of COX-2, MMP-9 and p53 were sequentially 61.5%, 42.9% and 50.5%. In staging, distant metastasis, therapeutic modalities, there was no significant differences between two histologic types of NSCLC. Interestingly, expression of COX-2 gene was higher in tissues of adenocarcinoma than squamous cell carcinoma(p=0.000). In expression of p53 and MMP-9, there was no statistically significant difference between two carcinoma groups.
Baseline clinical characteristics were similar between early and advanced carcinoma. Rate of curative resection for patients with early cancer was 76.5% and significantly higher than for patients with advanced stage(p=0.000). Accordingly, duration of overall survival was longer in early stage than in advanced stage(p=0.001). Expression of three genes between early versus advanced NSCLC was similar. But, in stage IV NSCLC, two cases with grade III positive staining for COX-2 and 1 case grade II positive for MMP-9, which were the strongest staining among total cases.
Between early stage of carcinoma and advanced diseases, there was no significant difference in expression of COX-2, MMP-9 and p53. In 28.1% of squamous cell carcinoma and 5.8% of adenocarcinoma showed no expression of COX-2, MMP-9 and p53, statistical difference of which was significant(p=0.025, Table 4).
Median duration of overall survival was similar among these groups. Notably, survival duration was longer in the group with positive expression of p53 and negative for COX-2 and MMP-9(median duration of survival = 165.6 weeks).
MMP-9 was significantly associated with expression of COX-2(p=0.006, Table 5A). Seventy five percent of patients with negative stain for COX-2 were also negative in MMP-9. Among patients with positive stain for MMP-9, 78.6% patients were positive stain for COX-2. In squamous cell carcinoma, COX-2 expression was correlated significantly with expression of MMP-9 (p=0.007), and also seemed to be correlated with mutant type p53 (p=0.061) (Table 5B).
With the use of Cox's proportional hazards model, COX-2 expression was significant prognostic factor for survival in resectable cancer group (relative risk = 0.591; 95% confidence interval 0.383 - 0.913; pp=0.018: Table 6A). In unresectable NSCLC group with curative resection, MMP-9 was statistically significant prognostic factor for overall survival (relative risk = 2.371; 95% confidence interval 1.044 - 5.388; pp=0.018: Table 6B).
Considering all carcinoma cases, prognostic factor for overall survival curative resection factor was only significant statistically (relative risk = 0.305; 95% confidence interval 0.173 - 0.536; p=0.000: Table 6C).
As the results of univariate analysis for all the study patients, significant predictors for survival were tumor stage, patient's age, curative resection, albumin, FEV1 (Table 6C) but not COX-2, MMP-9 and p53.
Curative resection was the only statistically significant prognostic factor for overall survival according to multivariate regressional analysis (relative risk = 0.482; 95% confidence interval 0.262 - 0.888; p=0.019: Table 6D).
In cases with curative resection, survival duration was significantly prolonged than those without surgery (median duration of survival in resected group versus that in inoperable group: 170.3 weeks vs. 57.7 weeks, p=0.0000, Figure 2A).
Median duration of survival in metastasis was 45.4 weeks, which was significantly shorter than 93.3 weeks in cases without metastasis (p=0.0009, Figure 2B). In cases of advanced carcinoma, median survival duration was 62.7 weeks, which was significantly shortened comparing 170.3 weeks in early carcinoma group (p=0.0028, Figure 2C). As prognostication results based on the expression of COX-2, MMP-9 and p53 among total study patients, there wasere no significant differences between positive and negative expression group (Figure 2D).
We have investigated the expression patterns and their prognostic roles of COX-2, MMP-9 and p53 in NSCLC. Considering expressional patterns of genes, MMP-9 was associated with COX-2. COX-2 expression is suppressed by wild type p53 but not by mutant p53. In a study of squamous cell carcinoma in head and neck, authors showed no relation between two genes34, we could find that COX-2 and mutant p53 were slightly significant in squamous cell carcinoma. COX-2 was more frequently expressed in adenocarcinoma than in squamous cell carcinoma. In squamous cell carcinoma, negative expression for COX-2, MMP-9 and p53 was more frequently observed than adenocarcinoma.
In our study, according to tumor staging, these gene expressions were similar patterns. Curative resection, tumor staging and metastasis were absolute prognostic factors for survival of NSCLC as results of this study, which were compatible to previous studies.
Based on total study patients, overexpression of these three genes in NSCLC had limited role as prognostic factors. But in subgroup analysis, COX-2 had prognostic significance in survival of curatively resected NSCLC groups. And MMP-9 had significantly affected on survival of inoperable cases. Overall curative resection for NSCLC is most important factor, but further study for prognostication of expression of COX-2 and other genes seems to be very important.
Some studies showed that increased COX-2 expression was related with shortened survival only in patients with stage I and II NSCLC35,36.
MMP expression in NSCLC was variable: some MMP increased, others unchanged and also some MMP decreased37. The abnormal circulatory pattern of MMP-9 was found in advanced lung cancer38.
Yamaguchi et al suggested that the combination of COX-2 and MMP-9 and categorizing tumors would be important prognostic factors in patients with resected adenocarcinoma of the lung39. Tumor staining for MMP-9 in resected adenocarcinoma of the lung is strongly related to survival40.
As notable results from this study, significant correlation was found between expression of MMP-9 and COX-2. COX-2 expression was significant prognostic factor for survival in resected cancer group. In inoperable non-small cell lung cancer group, MMP-9 was statistically significant prognostic factor for overall survival.
COX-2 and MMP-9 expression may have some important significance in pathogenesis and prognosis in non-small cell lung cancer, especially in adenocarcinoma.
This is retrospective study. Inaccurate clinical factors were not included for prognostic analysis ??i.e., clinical performance status. Selection bias for cases may exist. Adenocarcinoma and squamous cell carcinoma were included as for non-small cell lung cancer.
Evidence for COX-2 and MMP-9 expression in NSCLC was proved in this study. So further precise mechanism of tumorigenic and metastatic activity in NSCLC reserves to be validated with other research tools and with more study populations.
Taken together, COX-2 and MMP-9 might have some roles for progression or prognosis in some selected patients with non-small cell lung cancer. COX-2 and MMP-9 may have some roles for disease progression or prognosis in selected patients with NSCLC.
Figures and Tables
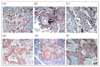 | Figure 1Overexpression of COX-2 (A, D), MMP-9 (B, E) and p53 (C, F) in Adenocarcinoma (A, B, C) and Squamous cell carcinoma (D, E, F) of the lungs with immunohistochemical staining (×100). |
 | Figure 2A. Survival analysis of all study patients according to curative resection. Median survival duratrion in resected cases was 170.3 weeks (95% CI ; 109.1 - 231.5 weeks), which was more prolonged from that in inoperable cases [57.7 weeks (95% CI ; 39.6 - 75.8 weeks)](p=0.0000;Log rank test)
B. Survival analysis of all study patients according to metastasis. Median survival duration in 71 cases without metastasis was 93.3 weeks (95% CI; 55.1 - 131.5 weeks) was superior to that in 20 cases with metastasis [45.4 weeks (95% CI; 15.9 - 74.9 weeks)] (p=0.0009; Log rank test).
C. According to stage, median survival duration of 34 cases with early stage (stage I,II) was 170.3 weeks (95% CI; 107.2 -233.4 weeks), which was significantly longer than survival duration of 57 cases with advanced lung cancers [62.7 weeks (95% CI; 45.0 - 80.5 weeks)] (p=0.0028; Log rank test).
D. Survival curves according to the expression of COX-2, MMP-9 and p53. According to COX-2 expression, positive group seemed to have shorter survival duration (88.0 weeks) than that of negative group (57.7 weeks)(p=0.08). There were no significant differences between positive and negative expression between positive versus negative group based on the expression of MMP-9 and p53.
|
Table 2
Clinical characteristics and immunohistochemical staining between adenocarcinoma and squamous cell carcinoma in NSCLC.

Table 3
Baseline clinical characteristics and immunohistochemical expression of COX-2, MMP-9, p53 in NSCLC according to early (stage I, II) and advanced stage (stage III, IV)

References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
2. Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998. 396:643–649.
3. Lee JS, Kim SY, Hong WK, Lippman SM, Ro JY, Gay ML, et al. Detection of chromosomal polysomy in oral leukoplakia, a premalignant lesion. J Natl Cancer Inst. 1993. 85:1951–1954.
4. Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosomes Cancer. 1999. 25:195–204.
5. Phillips JL, Hayward SW, Wang Y, Vasselli J, Pavlovich C, Padilla-Nash H, et al. The consequences of chromosomal aneuploidy on gene expression profiles in a cell line model for prostate carcinogenesis. Cancer Res. 2001. 61:8143–8149.
6. Hittelman WN. Genetic instability in epithelial tissues at risk for cancer. Ann N Y Acad Sci. 2001. 952:1–12.
7. Mao L, Lee DJ, Tockman MS, Erozan YS, Askin F, Sidransky D. Microsatellite alterations as clonal markers for the detection of human cacner. Proc Natl Acad Sci U S A. 1994. 91:9871–9875.
8. Pifarre A, Rosell R, Monzo M, De Anta JM, Moreno I, Sanchez JJ, et al. Prognostic value of replication errors on chromosomes 2p and 3p in non-small cell lung cancer. Br J Cancer. 1997. 75:184–189.
9. Field JK, Neville EM, Stewart MP, Swift A, Liloglou T, Risk JM, et al. Fractional allele loss data indicate distinct genetic populations in the development of non-small cell lung cancer. Br J Cancer. 1996. 74:1968–1974.
10. Rosell R, Pifarre A, Monzo M, Astudillo J, Lopez-Cabrerizo MP, Calvo R, et al. Reduced survival in patients with stage-I non-small-cell lung cancer associated with DNA-replication errors. Int J Cancer. 1997. 74:330–334.
11. Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell carcinoma. Oncogene. 1999. 18:643–650.
12. Zhou X, Kemp BL, Khuri FR, Liu D, Lee JJ, Wu W, et al. Prognostic implications of microsatellite alteration profiles in early-stage non-small cell lung cancer. Clin Cancer Res. 2000. 6:559–565.
13. Smith AL, Hung J, Walker L, Rogers TE, Vuitch F, Lee E, et al. Extensive areas of aneuploidy are present in the respiratory epithelium of lung cancer patients. Br J Cancer. 1996. 73:203–209.
14. Slaughter D, Southwick H, Smejkal W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1953. 6:963–968.
15. Lee JJ, Liu D, Lee JS, Kurie JM, Khuri FR, Ibarguen H, et al. Long-term impact of smoking on lung epithelial proliferation in current and former smokers. J Natl Cancer Inst. 2001. 93:1081–1088.
16. Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999. 29:688–696.
17. Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999. 46:407–412.
18. Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998. 19:723–729.
19. Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer : a population-based study. Arch Intern Med. 1999. 159:161–166.
20. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Upregualtion of cyclooxygenase 2 gene expression human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994. 107:1183–1188.
21. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Kato H, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995. 55:3785–3789.
22. Riedl K, Krysan K, Pold M, Dalwadi H, Heuze-Vourc'h N, Dohadwala M, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat. 2004. 7:169–184.
23. Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003. 22:9030–9040.
24. Adimoolam S, Ford JM. p53 and regulation of DNA damage recognition during nucleotide excision repair. DNA Repair (Amst). 2003. 2:947–954.
25. Smith ML, Seo YR. p53 regulation of DNA excision repair pathways. Mutagenesis. 2002. 17:149–156.
26. Pollan M, Varela G, Torres A, de la Torre M, Ludena MD, Ortega MD, et al. Clinical value of p53, c-erbB2, CEA and CA125 regrding relapse, metastasis and death in resectable non-small cell-lung cancer. Int J Cancer. 2003. 107:781–790.
27. Leversha MA, Fielding P, Watson S, Gosney JR, Field JK. Expression of p53, pRB, and p16 in lung tumors: a validation study on tissue microarrays. J Pathol. 2003. 200:610–619.
28. Kwiatkowski DJ, Harpole DH Jr, Godleski J, Herndon JE 2nd, Shieh DB, Richards W, et al. Molecular pathologic substaging in 244 stage I non-small-cell lung cancer patients: clinical implications. J Clin Oncol. 1998. 16:2468–2477.
29. Osaki T, Oyama T, Inoue M, Gu CD, Kodate M, Aikawa M, et al. Molecular biologic markers and micrometastasis in resected non-small cell lung cancer. Prognostic implications. Jpn J Thorac Cardiovasc Surg. 2001. 49:545–551.
30. Geradts J, Fong KM, Zimmerman PV, Maynard R, Minna JD. Correlation of abnormal p16INK4A, and p53 expression with 3p loss of heterozygosity, other genetic abnormalities, and clinical features in 103 primary non-small cell lung cancers. Clin Cancer Res. 1999. 5:791–800.
31. Zereu M, Vinholes JJ, Zettler CG. P53 and Bcl-2 protein expression and its relationship with prognosis in small-cell lung cancer. Clin Lung Cancer. 2003. 4:298–302.
32. Sakuma K, Fujimori T, Hirabayashi K, Terano A. Cyclooxygenase (COX)-2 immunoreactivity and relationship to p53 and Ki-67 expression in colorectal cancer. J Gastroenterol. 1999. 34:189–194.
33. Shattuck-Brandt RL, Lamps LW, Heppner Goss KJ, DuBois RN, Matrisan LM. Differential expression of matrilysin and cyclooxygenase-2 in intestinal and colorectal neoplasms. Mol Carcinog. 1999. 24:177–187.
34. Atula T, Hedstrom J, Ristimaki A, Finne P, Leivo I, Markkanen-Leppanen M, et al. Cyclooxygenase-2 expression in squamous cell carcinoma of the oral cavity and pharynx: association to p53 and clinical outcome. Oncol Rep. 2006. 16:485–490.
35. Laga AC, Zander DS, Cagle PT. Prognostic significance of cyclooxygenase 2 expression in 259 cases of non-small cell lung cancer. Arch Pathol Lab Med. 2005. 129:1113–1117.
36. Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001. 7:861–867.
37. Lin TS, Chiou SH, Wang LS, Huang HH, Chiang SF, Shih AY, et al. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep. 2004. 12:717–723.
38. Jumper C, Cobos E, Lox C. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Respir Med. 2004. 98:173–177.
39. Yamaguchi NH, Lichtenfels AJ, Demarchi LM, da Silva AP, Garippo AL, Alves VF, et al. COX-2, MMP-9, and Noguchi classification provide additional prognostic information about adenocarcinoma of the lung. A study of 117 patients from Brazil. Am J Clin Pathol. 2004. 121:78–86.
40. Pinto CA, Carvalho PE, Antonangelo L, Garippo A, Da Silva AG, Soares F, et al. Morphometric evaluation of tumor matrix metalloproteinase 9 predicts survival after surgical resection of adenocarcinoma of the lung. Clin Cancer Res. 2003. 9:3098–3104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download